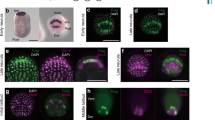
Abstract
IN Drosophila the amount of neurogenic ectoderm, from which the central nervous system (CNS) derives, is regulated by a dorsal-ventral system of positional information in which two secreted molecules of antagonistic functions, decapentaplegic (dpp) and short-gastrulation (sog), play fundamental roles1-4. The vertebrate homologue of dpp is either bmp-4 or bmp-2 (ref. 5), and the homologue of sog is chd 4,6,7 (s-chordin). In Xenopus the CNS is induced by signals emanating from the organizer8, and two proteins secreted by the organizer, noggin9 and follistatin10, have been shown to induce neural tissue in animal-cap assays. Here we report that Chd, another organizer-specific secreted factor6, has neuralizing activity and that this activity can be antagonized by Bmp-4. Inhibition of the function of the endogenous Bmp-4 present in the animal cap11 also leads to neural differentiation. We suggest that conserved molecular mechanisms involving chd/sog and bmp-4/fdpp gene products pattern the ectoderm in Xenopus and in Drosophila.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
01 November 1995
A Correction to this paper has been published: https://doi.org/10.1038/378419d0
References
Ferguson, E. L. & Anderson, K. V. Cell 71, 451–461 (1992).
Ferguson, E. L. & Anderson, K. V. Development 114, 583–597 (1992).
François, V., Solloway, M., O'Neill, J. W., Energy, J. & Bier, E. Genes Dev. 8, 2602–2616 (1994).
Holley, S. A. et al. Nature 376, 249–253 (1995).
Padgett, R. W., St Johnson, R. D. & Gelbart, W. M. Proc. natn. Acad. Sci. U.S.A. 90, 2905–2909 (1994).
Sasai, Y. et al. Cell 79, 779–790 (1994).
François, V. & Bier, E. Cell 80, 19–20 (1995).
Hamburger, V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. (Oxford Univ. Press, New York. 1988).
Lamb, T. M. et al. Science 262, 713–718 (1993).
Hemmati-Birvanlou, A., Kelly, O. G. & Melton, D. A. Cell 77, 283–295 (1994).
Fainsod, A., Steinbeisser, H. & De Robertis, E. M. EMBO J. 13, 5015–5025 (1994).
Niehrs, C., Keller, R., Cho, K. W. Y. & De Robertis, E. M. Cell 72, 491–503 (1993).
Ruiz i Altaba, A., Cox, C., Jessell, T. M. & Klar, A. Proc. natn. Acad. Sci. U.S.A. 90, 8268–8272 (1993).
Dale, L., Howes, G., Price, B. M. J. & Smith, J. C. Development 115, 573–585 (1992).
Jones, C. M., Lyons, K. M., Lapan, P. M., Wright, C. V. E. & Hogan, B. L. M. Development 115, 639–647 (1992).
Richter, K., Grunz, H. & Dawid, I. B. Proc. natn. Acad. Sci. U.S.A. 85, 8086–8090 (1988).
Graff, J. M., Thies, S. R., Song, J. J., Celeste, A. J. & Melton, D. A. Cel 79, 169–179 (1994).
Suzuki, A. et al. Proc. natn. Acad. Sci. U.S.A, 91, 10255–10259 (1994).
Schulte-Merker, S., Smith, J. C. & Dale, L. EMBO J. 13, 3533–3541 (1994).
Matzuk, M. M. et al. Nature 374, 360–363 (1995).
Shawlot, W. & Behringer, R. R. Nature 374, 425–430 (1995).
Taira, M., Otani, H., Saint-Jeannet, J. P. & Dawid, I. B. Nature 372, 677–699 (1994).
Hemmati-Brivanlou, A. & Melton, D. A. Nature 359, 609–614 (1992).
Hemmati-Brivanlou, A. & Melton, D. A. Cell 77, 273–281 (1994).
Frasch, M. Nature 374, 464–467 (1995).
Sharpe, C. R., Pluck, A. & Gurdon, J. B. Development 107, 701–714 (1989).
Jamrich, M. & Sato, S. Development 105, 779–786 (1989).
Zaraisky, A. G. et al. Devl Biol. 152, 373–382 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sasai, Y., Lu, B., Steinbeisser, H. et al. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333–336 (1995). https://doi.org/10.1038/376333a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/376333a0
This article is cited by
-
Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: from Neural Stem Cells to Cerebral Organoids
Stem Cell Reviews and Reports (2022)
-
Foxd4l1.1 negatively regulates transcription of neural repressor ventx1.1 during neuroectoderm formation in Xenopus embryos
Scientific Reports (2020)
-
Hindbrain induction and patterning during early vertebrate development
Cellular and Molecular Life Sciences (2019)
-
Time-Course Gene Expression Profiling Reveals a Novel Role of Non-Canonical WNT Signaling During Neural Induction
Scientific Reports (2016)
-
Epigenetic regulation of early neural fate commitment
Cellular and Molecular Life Sciences (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.