Abstract
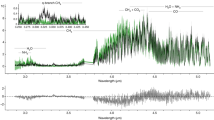
HETEROGENEOUS reactions on polar stratospheric clouds (PSCs) play a key role in the photochemical mechanism thought to be responsible for ozone depletion in the Antarctic and the Arctic1,2. Reactions on PSC particles activate chlorine to forms that are capable of photochemical ozone destruction, and sequester nitrogen oxides (NOx) that would otherwise deactivate the chlorine3,4. Although the heterogeneous chemistry is now well established, the composition of the clouds themselves is uncertain. It is commonly thought that they are composed of nitric acid trihydrate3, although observations have left this question unresolved5–14. Here we reanalyse infrared spectra of type I PSCs obtained in Antarctica in September 198715,16, using recently measured optical constants of the various compounds that might be present in PSCs17. We find that these PSCs were not composed of nitric acid trihydrate but instead had a more complex composition, perhaps that of a ternary solution. Because cloud formation is sensitive to their composition, this finding will alter our understanding of the locations and conditions in which PSCs form. In addition, the extent of ozone loss depends on the ability of the PSCs to remove NOx permanently through sedimentation. The sedimentation rates depend on PSC particle size which in turn is controlled by the composition and formation mechanism14.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Solomon, S. Nature 347, 347–354 (1990).
Toon, O. B. & Turco, R. P. Scient. Amer. 264, 68–74 (1991).
Toon, O. B., Hamill, P., Turco, R. P. & Pinto, J. Geophys. Res. Lett. 13, 1284–1287 (1986).
Schoeberl, M. R. et al. Geophys. Res. Lett. 20, 2511–2514 (1993).
Dye, J. E. et al. J. geophys. Res. 97, 8015–8034 (1992).
Kawa, S. R. et al. J. geophys. Res. 97, 7925–7938 (1992).
Browell, E. V. et al. Geophys. Res. Lett. 17, 385–388 (1990).
Rosen, J. M., Oltmans, S. J. & Evans, W. F. Geophys. Res. Lett. 16, 791–794 (1989).
Worsnop, D. R., Fox, L. E., Zahniser, M. S. & Wofsy, S. C. Science 259, 71–74 (1993).
Hanson, D. R. Geophys. Res. Lett. 17, 421–424 (1990).
Toon, O. B. et al. Science 261, 1136–1140 (1993).
Molina, M. J. et al. Science 261, 1418–1423 (1993).
Carslaw, K. S. et al. Geophys. Res. Lett. 21, 2479–2482 (1994).
Tabazadeh, A. et al. Geophys. Res. Lett. 21, 1619–1622 (1994).
Kinne, S. et al. J. geophys. Res. 94, 16481–16491 (1988).
Toon, G. C. et al. J. geophys. Res. 94, 16571–16596 (1989).
Toon, O. B., Tolbert, M. A., Koehler, B. G., Middlebrook, A. M. & Jordan, J. J. geophys. Res. 99, 25631–25654 (1994).
Crutzen, P. J. & Arnold, F. Nature 324, 651–655 (1986).
McElroy, M. B., Salawitch, R. J. & Wofsy, S. C. Geophys. Res. Lett. 13, 1296–1299 (1986).
Fahey, D. W. et al. J. geophys. Res. 94, 11299–11315 (1989).
Pueschel, R. F. et al. J. geophys. Res. 94, 11271–11284 (1989).
Hanson, D. & Mauersberger, K. Geophys. Res. Lett. 15, 855–858 (1988).
Gandrud, B. W. et al. J. geophys. Res. 94, 11285–11297 (1989).
Hofmann, D. J., Rosen, J. M., Harder, J. W. & Hereford, J. V. J. geophys. Res. 94, 11253–11270 (1989).
Koehler, B. G., Middlebrook, A. M. & Tolbert, M. A. J. geophys. Res. 97, 8065–8074 (1992).
Querry, M. R. & Tyler, I. L. J. chem. Phys. 72, 2495–2499 (1980).
Zhang, R., Wooldridge, P. J., Abbatt, J. P. D. & Molina, M. J. J. phys. Chem. 97, 7351–7358 (1993).
Pitari, G. & Ricciardulli, L. Geophys. Res. Lett. 21, 1791–1794 (1994).
Toon, O. B., Turco, R. P. & Hamill, P. Geophys. Res. Lett. 17, 445–448 (1990).
Peter, Th., Bruhl, C. & Crutzen, P. J. Geophys. Res. Lett. 18, 1465–1468 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toon, O., Tolbert, M. Spectroscopic evidence against nitric acid trihydrate in polar stratospheric clouds. Nature 375, 218–221 (1995). https://doi.org/10.1038/375218a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/375218a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.