Abstract
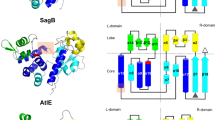
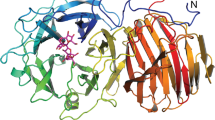
THE integrity of the bacterial cell wall depends on the balanced action of several peptidoglycan (murein) synthesizing and degrading enzymes1,2. Penicillin inhibits the enzymes responsible for pep-tide crosslinks in the peptidoglycan polymer3. Enzymes that act solely on the glycosidic bonds are insensitive to this antibiotic, thus offering a target for the design of antibiotics distinct from the β-lactams. Here we report the X-ray structure of the periplasmic soluble lytic transglycosylase (SLT; Mr 70,000) from Escherichia coli This unique bacterial exomuramidase cleaves the β-l,4-glycosidic bonds of peptidoglycan to produce small 1,6-anhydro-muropeptides4–6. The structure of SLT reveals a 'superhelicaP ring of α-helices with a separate domain on top which resembles the fold of lysozyme. Site-directed mutagenesis and a crystallographic inhibitor-binding study confirmed that the lysozyme-like domain contains the active site of SLT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rogers, H. J., Perkins, H. R. & Ward, J. B. in Microbial Cell Walls and Membranes (eds Perkins, H. R. et al.) 437–460 (Chapman & Hall, London, 1980).
Höltje, J.-V. & Schwarz, U. in Molecular Cytology of Escherichia coli (ed. Nanninga, N.) 77–119 (Academic, London, 1985).
Waxman, D. J. & Strominger, J. L. A. Rev. Biochem. 52, 825–869 (1983).
Höltje, J.-V., Mirelman, D., Sharon, N. & Schwarz, U. J. Bact. 124, 1067–1076 (1975).
Keck, W., Wientjes, F. B. & Schwarz, U. Eur. J. Biochem. 148, 493–497 (1985).
Engel, H., Kazemier, B. & Keck, W. J. Bact. 173, 6773–6782 (1991).
Presnell, S. R. & Cohen, F. E. Proc. natn. Acad. Sci. U.S.A. 86, 6592–6595 (1989).
Banaszak, L., Sharrock, W. & Timmins, P. A. Rev. Biophys. biophys. Chem. 20, 221–246 (1991).
Barlow, D. J. & Thornton, J. M. J. molec. Biol. 201, 601–619 (1988).
Verschueren, K. H. G., Franken, S. M., Rozeboom, H. J., Kalk, K. H. & Dijkstra, B. W. J. molec. Biol. 232, 856–872 (1993).
Imoto, T., Johnson, L. N., North, A. C. T., Phillips, D. C. & Rupley, J. A. in The Enzymes (ed. Boyer, P. D.) 666–868 (Academic, New York, 1972).
Weaver, L. H. & Matthews, B. W. J. molec. Biol. 193, 189–199 (1987).
Blake, C. C. F. et al. Proc. R. Soc. Lond. B167, 378–388 (1967).
Hardy, L. W. & Poteete, A. R. Biochemistry 30, 9457–9463 (1991).
Strynadka, N. C. J. & James, M. N. G. J. molec. Biol. 220, 440–424 (1991).
Beachey, E. H., Keck, W., de Pedro, M. A. & Schwarz, U. Eur. J. Biochem. 116, 355–358 (1981).
Rozeboom, H. J., Dijkstra, B. W., Engel, H. & Keck, W. J. molec. Biol. 212, 557–559 (1990).
Messerschmidt, A. & Pflugrath, J. W. J. appl. Crystallogr. 20, 306–315 (1987).
Kabsch, W. J. appl. Crystallogr. 21, 916–924 (1988).
Bricogne, G. Acta crystallogr. A 32, 832–847 (1976).
Jones, T. A. J. appl. Crystallogr. 11, 268–272 (1978).
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Brünger, A. T. X-PLOR (Version 3.0) Manual (Yale Univ., New Haven, 1992).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Rossmann, M. G. & Argos, P. J. molec. Biol. 105, 75–96 (1976).
Bernstein, F. C. et al. J. molec. Biol. 112, 535–542 (1977).
Shinagawa, S., Maki, M., Kintaka, K., Imada, A. & Asai, M. J. Antibiot. 38, 17–23 (1985).
Templin, M. F., Edwards, D. H. & Höltje, J.-V. J. biol. Chem. 267, 20039–20043 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thunnissen, AM., Dijkstra, A., Kalk, K. et al. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature 367, 750–753 (1994). https://doi.org/10.1038/367750a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/367750a0
This article is cited by
-
RNase III Controls mltD mRNA Degradation in Escherichia coli
Current Microbiology (2014)
-
The PECACE domain: a new family of enzymes with potential peptidoglycan cleavage activity in Gram-positive bacteria
BMC Genomics (2005)
-
Subcellular Location of the Soluble Lytic Transglycosylase Homologue in Staphylococcus aureus
Current Microbiology (2005)
-
Clathrin self-assembly is mediated by a tandemly repeated superhelix
Nature (1999)
-
Chitinases, chitosanases, and lysozymes can be divided into procaryotic and eucaryotic families sharing a conserved core
Nature Structural Biology (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.