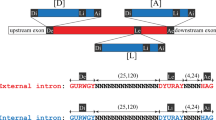
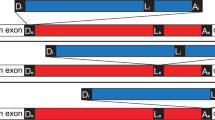
Abstract
LIKE nuclear premessenger introns, group II self-splicing introns are excised from primary transcripts as branched molecules, containing a 2′–5′ phosphodiester bond. For this reason, it is widely believed that the ribozyme (catalytic RNA) core of group II introns, or some evolutionarily related molecule, gave rise to the RNA components of the spliceosomal splicing machinery of the eukaryotic nucleus1. One difficulty with this hypothesis has been the restricted distribution of group II introns. Unlike group I self-splicing introns, which interrupt not only organelle primary transcripts, but also some bacterial and nuclear genes2–5, group II introns seemed to be confined to mitochondrial and chloroplast genomes (reviewed in ref. 6). We now report the discovery of group II introns both in cyanobacteria (the ancestors of chloroplasts7) and the γ subdivision of purple bacteria, or proteobacteria8, whose α subdivision probably gave rise to mitochondria9. At least one of these introns actually self-splices in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sharp, P. A. Science 254, 663 (1991).
Cech, T. R. Gene 73, 259–271 (1988).
Xu, M. Q., Kathe, S. D., Goodrich-Blair, H., Nierzwicki-Bauer, S. A. & Shub, D. A. Science 250, 1566–1570 (1990).
Kuhsel, M. G., Strickland, R. & Palmer, J. D. Science 250, 1570–1573 (1990).
Reinhold-Hurek, B. & Shub, D. A. Nature 357, 173–176 (1992).
Michel, F., Umesono, K. & Ozeki, H. Gene 82, 5–30 (1989).
Schwartz, R. M. & Dayhoff, M. O. Science 199, 395–403 (1978).
Stackebrandt, E., Murray, R. G. E. & Trüper, H. G. Int. J. syst. Bact. 38, 321–325 (1988).
Yang, D., Oyaizu, Y., Oyaizu, H., Olsen, G. & Woese, C. R. Proc. natn. Acad. Sci. U.S.A. 82, 4443–4447 (1985).
Michel, F. & Lang, B. F. Nature 316, 641–643 (1985).
Xiong, Y. & Eickbush, T. H. EMBO J. 9, 3353–3362 (1990).
Woese, C. R. et al. System, appl. Microbiol. 6, 25–33 (1985).
Dujon, B. Gene 82, 91–114 (1989).
Meunier, B., Tian, G. L., Macadre, C., Slonimski, P. P. & Lazowska, J. in Structure, Function and Biogenesis of Energy Transfer Systems (eds Quagliariello, E., Papa, S., Palmieri, F. & Saccone, C.) 169–174 (Elsevier, Amsterdam, 1990).
Skelly, P. J., Hardy, C. M. & Clark-Walker, G. D. Curr. Genet. 20, 115–120 (1991).
Kück, U. Molec. gen. Genet. 218, 257–266 (1989).
Ochman, H. & Selander, R. K. J. Bact. 157, 690–693 (1984).
Ausubel, F. M. et al. Current Protocols in Molecular Biology (Wiley, New York, 1989).
Herdman, M., Delaney, S. F. & Carr, N. G. J. gen. Microbiol. 79, 233–237 (1973).
Jacquier, A. & Michel, F. J. molec. Biol. 213, 437–447 (1990).
Hiom, K. & Sedgwick, S. G. J. Bact. 173, 7368–7391 (1991).
Tiranti, V., Barat-Guéride, M., Bijl, J., DiDonato, S. & Zeviani, M. Nucleic Acids Res. 19, 4291 (1991).
Chase, J. W., Merrill, B. M. & Williams, K. R. Proc. natn. Acad. Sci. U.S.A. 80, 5480–5484 (1983).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. J. molec. Biol. 215, 403–410 (1990).
Michel, F. et al. Genes Dev. 6, 1373–1385 (1992).
Peebles, C. L., Benatan, E. J., Jarrell, K. A. & Perlman, P. S. Cold Spring Harbor Symp. quant. Biol. 52, 223–232 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferat, JL., Michel, F. Group II self-splicing introns in bacteria. Nature 364, 358–361 (1993). https://doi.org/10.1038/364358a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/364358a0
This article is cited by
-
Categorizing 161 plant (streptophyte) mitochondrial group II introns into 29 families of related paralogues finds only limited links between intron mobility and intron-borne maturases
BMC Ecology and Evolution (2023)
-
The genetic diversity of cereulide biosynthesis gene cluster indicates a composite transposon Tnces in emetic Bacillus weihenstephanensis
BMC Microbiology (2014)
-
Evolutionary Dynamics of the mS952 Intron: A Novel Mitochondrial Group II Intron Encoding a LAGLIDADG Homing Endonuclease Gene
Journal of Molecular Evolution (2011)
-
Recurrent invasion of mitochondrial group II introns in specimens of Pylaiella littoralis (brown alga), collected worldwide
Current Genetics (2008)
-
Introns and the origin of nucleus–cytosol compartmentalization
Nature (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.