Abstract
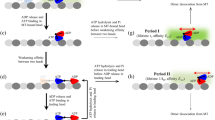
MOTOR proteins move unidirectionally along cytoskeletal polymers by coupling translocation to cycles of ATP hydrolysis. The energy from ATP is required both to generate force and to dissociate the motor–filament complex in order to begin a new Chemomechanical cycle1,2. For myosin, force production is associated with phosphate release following ATP hydrolysis, whereas dissociation of actomyosin is tightly coupled to the binding of ATP3. Dynein, a microtubule motor, uses a similar cycle4, suggesting that all cytoskeletal motors might operate by a common mechanism. Here we investigate kinesin's Chemomechanical cycle by assaying microtubule movement by single kinesin molecules when intermediate states in the hydrolysis cycle are prolonged with ATP analogues or inhibitors. In contrast to myosin and dynein, kinesin with bound ADP dissociates from microtubules during translocation, whereas kinesin with unhydrolysed nucleotide remains tightly associated with the polymer. These findings imply that kinesin converts ATP energy into mechanical work by a pathway distinct from that of myosin or dynein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Eisenberg, E. & Hill, T. L. Science 227, 999–1006 (1985).
Jencks, W. P. in The Roots of Modern Biochemistry 571–580 (Walter de Gruyer, Berlin, 1988).
Cooke, R. CRC crit. Rev. Biochem. 21, 53–118 (1986).
Johnson, K. A. A. Rev. Biophys. biophys. Chem. 14, 161–188 (1985).
Howard, J., Hudspeth, A. J. & Vale, R. D. Nature 342, 154–158 (1989).
Block, S. M., Goldtein, L. S. & Schnapp, B. J. Nature 348, 348–352 (1990).
Hackney, D. D. Proc. natn. Acad. Sci. U.S.A. 85, 6314–6318 (1988).
Pate, E. & Cooke, R. J. Musc. Res. Cell Motil. 12, 376–393 (1991).
Kuznetsov, S. A. & Gelfand, V. I. Proc. natn. Acad. Sci. U.S.A. 83, 8350–8354 (1986).
Goody, R. S. & Mannherz, H. G. in Protein-Ligand Interactions (eds Sund, H. & Blauer, G.) (Walter de Hruyer, Berlin, 1975).
Shimizu, T., Katsura, T., Domanico, P. L., Marchese-Ragona, S. P. & Johnson, K. A. Biochemistry 28, 7022–7027 (1989).
Cohn, S. A., Ingold, A. L. & Scholey, J. M. J. biol. Chem. 264, 4290–4297 (1989).
Lymm, R. W. & Taylor, E. W. Biochemistry 10, 4617–4624 (1971).
Porter, M. E. & Johnson, K. A. J. biol. Chem. 258, 6582–6587 (1983).
Omoto, C. K. & Johnson, K. A. Biochemistry 25, 419–427 (1986).
Sadhu, A. & Taylor, E. W. J. biol. Chem. 267, 11352–11359 (1992).
Taylor, E. W. in Heart and Cardiovascular System (eds Fozzard, H. A.) 1281–1293 (Raven, New York, 1992).
Holzbaur, E. L. F. & Johnson, J. A. Biochemistry 25, 428–434 (1986).
Mathies, R. A., Lin, S. W., Ames, J. B. & Pollard, W. T. A. Rev. Biophys. Chem. 20, 491–518 (1991).
Vale, R. D., Reese, T. S. & Sheetz, M. P. Cell 42, 39–50 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Romberg, L., Vale, R. Chemomechanical cycle of kinesin differs from that of myosin. Nature 361, 168–170 (1993). https://doi.org/10.1038/361168a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/361168a0
This article is cited by
-
Design principles governing chemomechanical coupling of kinesin
Scientific Reports (2017)
-
Network Complexity and Parametric Simplicity for Cargo Transport by Two Molecular Motors
Journal of Statistical Physics (2013)
-
Construction of a laser combiner for dual fluorescent single molecule imaging of pRNA of phi29 DNA packaging motor
Biomedical Microdevices (2010)
-
Energy Conversion by Molecular Motors Coupled to Nucleotide Hydrolysis
Journal of Statistical Physics (2009)
-
The role of casein in supporting the operation of surface bound kinesin
Journal of Biological Engineering (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.