Abstract
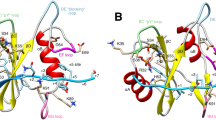
RECEPTOR protein-tyrosine kinases, through phosphorylation of specific tyrosine residues, generate high-affinity binding sites which direct assembly of multienzyme signalling complexes1,2. Many of these signalling proteins, including phospholipase Cγ, GTPase-activating protein and phosphatidylinositol-3-OH kinase, contain src-homology 2 (SH2) domains, which bind with high affinity and specificity to tyrosine-phosphorylated sequences3,4. The critical role played by SH2 domains in signalling has been highlighted by recent studies showing that mutation of specific phosphorylation sites on the platelet-derived growth factor receptor impair its association with phosphatidylinositol-3-OH kinase, preventing growth factor-induced mitogenesis5,6. Here we report the solution structure of an isolated SH2 domain from the 85K regulatory subunit of phosphatidylinositol-3-OH kinase, determined using multidimensional nuclear magnetic resonance spectroscopy. The structure is characterized by a central region of β-sheet flanked by two α-helices, with a highly flexible loop close to functionally important residues previously identified by site-directed mutagenesis7,8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ullrich, A. & Schlessinger, J. Cell 61, 203–212 (1990).
Cantley, C. L. et al. Cell 64, 281–302 (1991).
Koch, C. A. et al. Science 252, 668–674 (1991).
Pawson, T. Curr. Opinion struct. Biol. 2, 432–437 (1992).
Fantl, W. J. et al. Cell 69, 413–423 (1992).
Kashishian, A., Kazlauskas, A. & Cooper, J. A. EMBO J. 11, 1373–1382 (1992).
Mayer, B. J. et al. Molec. cell. Biol. 12, 609–618 (1992).
Mayer, B. J. & Hanafusa, H. J. Virol. 64, 3581–3589 (1990).
Otsu, M. et al. Cell 65, 91–104 (1991).
Hiles, I. et al. Cell (in the press).
Smith, D. B. & Johnson, K. S. Gene 67, 31–40 (1988).
Russell, R. B., Breed, J. & Barton, G. J. FEBS Lett. 304, 15–20 (1992).
Maniatis, T., Fritsch, E. F. & Sambrook, J. Molecular Cloning: A Laboratory Manual (Cold Spring Harbour Laboratory Press, New York, 1982).
Marion, D. et al. Biochemistry 28, 6150–6156 (1989).
Driscoll, P. C. et al. Biochemistry 29, 3542–3556 (1991).
Wüthrich, K. et al. FEBS Lett. 285, 327–347 (1991).
Wishart, D. S., Sykes, B. D. & Richards, F. M. Biochemistry 31, 1647–1651 (1992).
Brünger, A. T. et al. Protein Engng 1, 399–406 (1987).
Brünger, A. T. XPLOR Manual (Yale University, New Haven, 1988).
Nilges, M. et al. Protein Engng 2, 27–38 (1988).
Downing, A. K. et al. J. molec. Biol. 225, 821–833 (1992).
Nilges, M. et al. FEBS Lett 229, 317–324 (1988).
Brooks, B. R. et al. J. comp. Chem. 4, 187–217 (1983).
McGlade, C. J. et al. Molec. Cell. Biol. 12, 991–997 (1992).
Wüthrich, K. et al. J. molec. Biol. 169, 949–961 (1983).
Kay, L. E. & Bax, A. J. magn. Reson. 86, 110–126 (1990).
Güntert, P., Braun, W. & Wüthrich, K. J. molec. Biol. 217, 517–530 (1991).
Kraulis, P. J. J. Appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Booker, G., Breeze, A., Downing, A. et al. Structure of an SH2 domain of the p85α subunit of phosphatidylinositol-3-OH kinase. Nature 358, 684–687 (1992). https://doi.org/10.1038/358684a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358684a0
This article is cited by
-
1H, 13C, 15N chemical shift assignments of SHP2 SH2 domains in complex with PD-1 immune-tyrosine motifs
Biomolecular NMR Assignments (2020)
-
Recent advances on polyproline II
Amino Acids (2017)
-
Structure and regulation of Src family kinases
Oncogene (2004)
-
Structural basis for specificity of GRB2-SH2 revealed by a novel ligand binding mode
Nature Structural Biology (1996)
-
Crystal structure of the PI 3-kinase p85 amino-terminal SH2 domain and its phosphopeptide complexes
Nature Structural & Molecular Biology (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.