Abstract
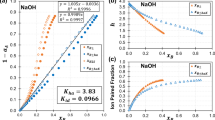
A QUANTITATIVE understanding of the rates and mechanisms of dissolution of crystalline solids in aqueous solutions is critical to the chemical modelling of many geochemical, environmental and industrial processes. Here I show that a linear free energy equation, developed recently1,2 for the prediction of the standard Gibbs free energies of formation of isostructural families of crystalline solids, can also be used for predicting the dissolution rates of solids. This equation bears a close analogy with the Hammett equation for aqueous organics3. Regression of data for the surface-reaction-controlled dissolution rates of isostructural families of divalent metal oxides and orthosilicates using the new equation yields coefficients characteristic of the specific crystal structure, whichturn out to be very close to the coefficients obtained by regression of standard free energy data for the same families. These results suggest that standard free energy coefficients can be used to predict dissolution rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sverjensky, D. A. & Molling, P. A. Nature 355, 231–234 (1992).
Sverjensky, D. A. Geol. Soc. Am. A. Meeting Abstr. 23, A212 (1991).
Wells, P. R. Linear Free Energy Relationships (Academic, London, 1968).
Exner, O. Correlation Analysis of Chemical Data (Plenum, New York, 1988).
Brezonik, P. L. in Aquatic Chemical Kinetics (ed. Stumm, W.) 113–143 (Wiley, New York, 1990).
Hammett, D. J. Am. chem. Soc. 59, 96–103 (1937).
Casey, W. H. & Westrich, H. R. Nature 355, 157–159 (1992).
Casey, W. H. J. Colloid. Interf. Sci. 146, 586–589 (1991).
Murphy, W. M. & Helgeson, H. C. Geochim. cosmochim. Acta. 51, 3137–3153 (1987).
Murphy, W. M. & Helgeson, H. C. Am. J. Sci. 289, 17–101 (1989).
Lasaga, A. C. in Kinetics of Geochemical Processes (eds Lasaga, A. C. & Kirkpatrick, R. J.) 261–319 (Mineralogical Society of America, Washington DC, 1981).
Stumm, W. & Wieland, E. in Aquatic Chemical Kinetics (ed. Stumm, W.) 367–400 (Wiley, New York, 1990).
Wieland, E., Wehrli, B. & Stumm, W. Geochim. cosmochim. Acta 52, 1969–1982 (1988).
Blum, A. E. & Lasaga, A. C. Geochim. cosmochim. Acta 55, 2193–2201 (1991).
Glushko, V. P. Thermal Constants of Substances (Viniti, Moscow, 1965–1981).
Berman, R. G. J. Petrol. 29, 445–522 (1988).
Robinson, G. R. et al. U.S. Dept. Int. Geol. Surv. Open File Rep. 83–72 (1982).
Robie, R. A., Hemingway, B. S. & Takei, H. Am. Mineral. 67, 470–482 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sverjensky, D. Linear free energy relations for predicting dissolution rates of solids. Nature 358, 310–313 (1992). https://doi.org/10.1038/358310a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358310a0
This article is cited by
-
Inhibitory Effect of MgO, FeO, CaF2, and Al2O3 Additives on the Dissolution Behavior of Ca from Silicate Mineral Phases into Water
Metallurgical and Materials Transactions B (2022)
-
Kinetic evaluation of sorption and desorption
Adsorption (2010)
-
Prediction of ligand-promoted dissolution rates from the reactivities of aqueous complexes
Nature (1995)
-
Physical surface-complexation models for sorption at the mineral–water interface
Nature (1993)
-
Direct scanning tunneling microscope (STM) observation of Cr(III) complexes on hematite (001) surfaces
Aquatic Sciences (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.