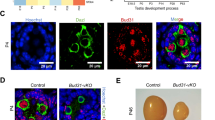
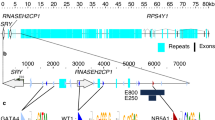
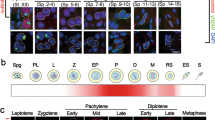
Abstract
MAMMALIAN spermatogenesis consists of a series of complex developmental processes controlled by the pituitary–hypothalamic axis1. This flow of biochemical information is directly regulated by the adenylate cyclase signal transaction pathway2. We have previously described the CREM (cyclic AMP-responsive element modulator) gene which generates, by cell-specific splicing, alternative antagonists of the cAMP transcriptional response3. Here we report the expression of a novel CREM isoform (CREMτ) in adult testis. CREMτ differs from the previously characterized CREM antagonists by the coordinate insertion of two glutamine-rich domains that confer transcriptional activation function. During spermatogenesis there was an abrupt switch in CREM expression. In premeiotic germ cells CREM is expressed at low amounts in the antagonist form. Subsequently, from the pachytene spermatocyte stage onwards, a splicing event generates exclusively the CREMτ activator, which accumulates in extremely high amounts. This splicing-dependent reversal in CREM function represents an important example of developmental modulation in gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Santen, R. J. in Endocrinology and Metabolism (eds Felig, P., Baxter, J. D., Broadus, A. E. & Frohman, L. A.) 821–905 (McGraw-Hill, New York, 1987).
Ewing, L. L. & Robaire, B. Ann. N.Y. Acad. Sci. 564, 1–302 (1989).
Foulkes, N. S., Borrelli, E. & Sassone-Corsi, P. Cell 64, 739–749 (1991).
Hoeffler, J. P., Meyer, T. E., Yun, Y., Jameson, J. L. & Habener, J. F. Science 242, 1430–1433 (1988).
Gonzalez, G. A. et al. Nature 337, 749–752 (1989).
Foulkes, N. S., Laoide, B. M., Schlotter, F. & Sassone-Corsi, P. Proc. natn. Acad. Sci. U.S.A. 88, 5448–5452 (1991).
Mellon, P. L., Clegg, C. H., Correll, L. A. & McKnight, G. S. Proc. natn. Acad. Sci. U.S.A. 86, 4887–4891 (1989).
Gonzales, G. A., Menzel, P., Leonard, J., Fischer, W. & Montminy, M. R. Molec. cell. Biol. 11, 1306–1312 (1991).
Courey, A. J. & Tjian, R. Cell 55, 887–898 (1989).
Lee, C. Q., Yun, Y., Hoeffler, J. P. & Habener, J. F. EMBO J. 9, 4455–4465 (1990).
Willison, K. & Ashworth, A. Trends Genet. 3, 351–355 (1987).
Dym, M. in Histology Cell and Tissue Biology (ed. Weiss, L.) 1000–1053 (Elsevier Biomedical, New York, 1983).
Lyon, M. F. & Hawkes, S. G. Nature 227, 1217–1219 (1970).
Moutier, R. in The Laboratory Animal in the Study of Reproduction (eds Antikatzides, T., Erichsen, S. & Spiegel, A.) 5–7 (Fischer, Stuttgart, 1976).
Beebe, S. J. et al. Molec. Endocrin. 4, 465–475 (1990).
McKnight, G. S. et al. Recent Prog. Horm. Res. 44, 307–335 (1988).
Auffray, C. & Rougeon, F. Eur. J. Biochem. 107, 303–314 (1980).
Sassone-Corsi, P., Ransone, L. J., Lamph, W. W. & Verma, I. M. Nature 336, 692–694 (1988).
Mather, J. P. Biol. Reprod. 23, 243–251 (1980).
Kilpatrick, D. L., Borland, K. & Jin, D. F. Proc. natn. Acad. Sci. U.S.A. 84, 5695–5699 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foulkes, N., Mellström, B., Benusiglio, E. et al. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature 355, 80–84 (1992). https://doi.org/10.1038/355080a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/355080a0
This article is cited by
-
PDE11A gene polymorphism in testicular cancer: sperm parameters and hormonal profile
Journal of Endocrinological Investigation (2021)
-
Analysis of mouse male germ cell-specific or -predominant Tex13 family genes encoding proteins with transcriptional repressor activity
Molecular Biology Reports (2021)
-
SOX30 is required for male fertility in mice
Scientific Reports (2017)
-
Knock-Out Serum Replacement and Melatonin Effects on Germ Cell Differentiation in Murine Testicular Explant Cultures
Annals of Biomedical Engineering (2017)
-
Alternative splicing: the pledge, the turn, and the prestige
Human Genetics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.