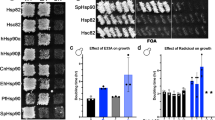
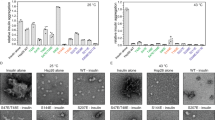
Abstract
MOST eukaryotic cells produce proteins with relative molecular masses in the range of 100,000 to 110,000 after exposure to high temperatures1. These proteins have been studied only in yeast and mammalian cells. In Saccharomyces cerevisiae, heat-shock protein hsp104 is vital for tolerance to heat, ethanol and other stresses (ref. 2, and Y.S. et al., manuscript submitted). The mammalian hsp110 protein is nucleolar and redistributes with growth state, nutritional conditions and heat shock3,5. The relationships between hsp110, hsp104 and the high molecular mass heat-shock proteins of other organisms were unknown. We report here that hsp104 is a member of the highly conserved ClpA/ClpB protein family first identified in Escherlchla coli6 and that additional heat-inducible members of this family are present in Schizosaccharomyces pombe and in mammals. Mutagenesis of two putative nucleotide-binding sites in hsp104 indicates that both are essential for function in thermotolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lindquist, S. & Craig, E. A. A. Rev. Genet. 22, 631–677 (1988).
Sanchez, Y. & Lindquist, S. L. Science 248, 1112–1115 (1990).
Subjeck, J. R., Shyy, T., Shen, J. & Johnson, R. J. J. Cell. Biol. 97, 1389–1395 (1983).
Shyy, T. T., Subjeck, J. R., Heinaman, R. & Anderson, G. Cancer Res. 46, 4738–4745 (1986).
Welch, W. J. & Suhan, J. P. J. Cell Biol. 101, 1198–1211 (1985).
Gottesman, S. et al. Proc. natn. Acad. Sci. U.S.A. 87, 3513–3517 (1990).
Brenner, S. Nature 334, 528–530 (1988).
Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. EMBO J. 1, 945–951 (1982).
Katayama-Fujimura, Y., Gottesman, S. & Maurizi, M. R. J. biol. Chem. 262, 4477–4487 (1987).
Hwang, B. J., Park, W. J., Chung, C. H. & Goldberg, A. L. Proc. natn. Acad. Sci. U.S.A. 84, 5550–5554 (1987).
Katayama, Y. et al. J. biol. Chem. 263, 15226–15236 (1988).
Hwang, B. J., Woo, K. M., Goldberg, A. L. & Chung, C. H. J. biol. Chem. 263, 8727–8734 (1988).
Gottesman, S., Clark, W. P. & Maurizi, M. R. J. biol. Chem. 265, 7886–7893 (1990).
Squires, C. L., Pedersen, S., Ross, B. M. & Squires, C. J. Bact. 173, 4254–4262 (1991).
Kitagawa, M., Wada, C., Yoshioka, S. & Yura, T. J. Bact. 173, 4247–4253 (1991).
Fry, D. C., Kuby, S. A. & Mildvan, A. S. Proc. natn. Acad. Sci. U.S.A. 83, 907–911 (1986).
Liu, Q. & Summers, W. C. Virology 163, 638–642 (1988).
Xia, Z. & Storm, D. R. J. biol. Chem. 265, 6517–6520 (1990).
Tanaka, K. et al. Biochem. biophys. Res. Commun. 164, 1253–1261 (1989).
Maurizi, M. R. et al. J. biol. Chem. 265, 12536–12545 (1990).
Hough, R., Pratt, G. & Rechsteiner, M. J. biol. Chem. 262, 8303–8313 (1987).
Eytan, E., Ganoth, D., Armon, T. & Hershko, A. Proc. natn. Acad. Sci. U.S.A. 86, 7751–7755 (1989).
Heinemeyer, W., Kleinschmidt, J. A., Saidowsky, J., Escher, C. & Wolf, D. H. EMBO J. 10, 555–562 (1991).
Emori, Y. et al. Molec. cell. Biol. 11, 344–353 (1991).
Finley, D., Ozkaynak, E. & Varshavsky, A. Cell 48, 1035–1046 (1987).
Seufert, W. & Jentsch, S. EMBO J. 9, 543–550 (1990).
Pelham, H. R. Cell 46, 959–961 (1986).
Gilman, M. in Current Protocols in Molecular Biology (ed. Ausubel, F. M.) 441–444 (Greene and Wiley-Interscience, New York, 1987).
Vijayraghavan, U., Company, M. & Abelson, J. Genes Dev. 3, 1206–1216 (1989).
Warrick, H. M., DeLozanne, A., Leinward, L. A. & Spudich, J. A. Proc. natn. Acad. Sci. U.S.A. 83, 9433–9437 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parselt, D., Sanchez, Y., Stitzel, J. et al. Hspl04 is a highly conserved protein with two essential nucleotide-binding sites. Nature 353, 270–273 (1991). https://doi.org/10.1038/353270a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/353270a0
This article is cited by
-
The molecular principles governing the activity and functional diversity of AAA+ proteins
Nature Reviews Molecular Cell Biology (2020)
-
AtHsp101 research sets course of action for the genetic improvement of crops against heat stress
Journal of Plant Biochemistry and Biotechnology (2020)
-
Effect of temperature on replicative aging of the budding yeast Saccharomyces cerevisiae
Biogerontology (2016)
-
Heat shock proteins: Molecules with assorted functions
Frontiers in Biology (2011)
-
[NSI +]: a novel non-Mendelian nonsense suppressor determinant in Saccharomyces cerevisiae
Current Genetics (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.