Abstract
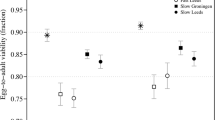
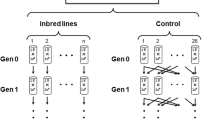
"THE effects of inbreeding may not be as noticeable in the first generation as the invigoration immediately apparent after crossing"1. This statement, published in 1919, has received little attention, and has apparently never been tested empirically, although the reduction of the genetic load of populations by inbreeding is well known in theoretical terms2–5. Because inbreeding increases homozygosity, and hence the effectiveness of selection against recessive or partially recessive detrimental alleles, changes in levels of inbreeding can lead to a reduction in the frequencies of such mutant alleles. This results in equilibration at higher population mean fitness6 and is referred to as 'purging' populations of their genetic load. Severe inbreeding can also reduce genetic load due to overdominant alleles, provided selection coefficients are not symmetrical at all loci, because alleles giving lower fitness will be reduced in frequency at equilibrium7,8. With either fitness model, however, reduction in genetic load takes time, and the initial effect of an increase in inbreeding is reduced fitness due to homozygosity. There are few data relating to the extent to which fitness is reduced during inbreeding in a set of lines and to how long the reduction lasts before increasing again to the initial level, or higher. Inbreeding experiments involving sib mating in mice and Drosophila subobscura10, and successive bottlenecks in house flies11 have yielded some evidence consistent with the purging hypothesis. Here, we report results of an experiment demonstrating a prolonged time-course of recovery of mean fitness under self-fertilization of a naturally outcrossing plant, and also compare our results with expectations derived by computer calculations. Our results show that the genetic load present in an outcrossing population can be explained only with a high mutation rate to partially recessive deleterious alleles, and that inbreeding purges the population of mutant alleles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
East, E. M. & Jones, D. F. Inbreeding and Outbreeding: Their Genetic and Sociological Significance 147 (Lippincott, Philadelphia, 1919).
Crow, J. F. Genetics 33, 477–487 (1948).
Wright, S. Evolution and the Genetics of Populations Vol. 3 (Univ. of Chicago Press, 1977).
Lande, R. & Schemske, D. W. Evolution 39, 24–40 (1985).
Charlesworth, D., Morgan, M. T. & Charlesworth, B. Evolution 44, 1469–1489 (1990).
Crow, J. F. in Mathematical Models in Population Genetics (eds Kojima, K.-I.) 128–177 (Springer-Verlag, Berlin, 1970).
Charlesworth, D. & Charlesworth, B. A. Rev. ecol. Syst. 18, 237–268 (1987).
Ziehe, M. & Roberds, J. H. Genetics 121, 861–868 (1989).
Falconer, D. S. Genet. Res. Camb. 17, 215–235 (1972).
Hollingsworth, M. J. & Maynard-Smith, J. J. Genet. 53, 295–314 (1955).
Bryant, E. H., Meffert, L. M. & McCommas, S. A. Amer. Nat. 138, 542–549 (1990).
Barrett, S. C. H. & Husband, B. C. Pl. Spec. Biol. 5, 41–55 (1990).
Glover, D. E. & Barrett, S. C. H. Heredity 59, 7–17 (1987).
Charlesworth, D. & Charlesworth, B. Evolution 44, 870–888 (1990).
Kondrashov, A. Nature 336, 435–440 (1988).
Charlesworth, B., Charlesworth, D. & Morgan, M. T. Nature 347, 380–382 (1990).
Shull, G. H. in Heterosis (ed. Gowen, J. W.) 14–48 (Iowa University Press, Ames, 1950).
Griffin, A. R. & Lindgren, D. Theor. appl. Genet. 71, 334–343 (1985).
Ohta, T. & Cockerham, C. C. Genet. Res. Camb. 23, 191–200 (1974).
Sing, C. F., Brewer, G. J. & Thirtle, B. Genetics 75, 381–404 (1973).
Brown, A. H. D. Theor. appl. Genet. 15, 1–42 (1979).
Templeton, A. R. & Read, B. Zoo Biol. 3, 177–199 (1984).
Wilton, A. N., Joseph, M. G. & Sved, J. Genet. Res. Camb. 54, 129–140 (1989).
Kondrashov, A. S. Genetics 111, 635–653 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barrett, S., Charlesworth, D. Effects of a change in the level of inbreeding on the genetic load. Nature 352, 522–524 (1991). https://doi.org/10.1038/352522a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/352522a0
This article is cited by
-
The impact of habitat loss and population fragmentation on genomic erosion
Conservation Genetics (2024)
-
Urbanization and a green corridor do not impact genetic divergence in common milkweed (Asclepias syriaca L.)
Scientific Reports (2023)
-
DNA barcode reveals high cryptic diversity in the commercially important Penaeini shrimps (Decapoda, Penaeidae)
Organisms Diversity & Evolution (2023)
-
Direct and correlated responses to artificial selection on foraging in Drosophila
Behavioral Ecology and Sociobiology (2023)
-
How likely was the successful introduction of the island canary to Midway Atoll?
Biological Invasions (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.