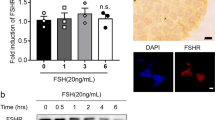
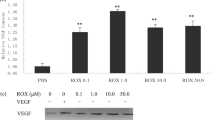
Abstract
The known endothelial mitogens stimulate growth of vascular endothelial cells without regard to their tissue of origin. Here we report a growth factor that is expressed largely in one type of tissue and acts selectively on one type of endothelium. This molecule, called endocrine-gland-derived vascular endothelial growth factor (EG-VEGF), induced proliferation, migration and fenestration (the formation of membrane discontinuities) in capillary endothelial cells derived from endocrine glands. However, EG-VEGF had little or no effect on a variety of other endothelial and non-endothelial cell types tested. Similar to VEGF, EG-VEGF possesses a HIF-1 binding site, and its expression is induced by hypoxia. Both EG-VEGF and VEGF resulted in extensive angiogenesis and cyst formation when delivered in the ovary. However, unlike VEGF, EG-VEGF failed to promote angiogenesis in the cornea or skeletal muscle. Expression of human EG-VEGF messenger RNA is restricted to the steroidogenic glands, ovary, testis, adrenal and placenta and is often complementary to the expression of VEGF, suggesting that these molecules function in a coordinated manner. EG-VEGF is an example of a class of highly specific mitogens that act to regulate proliferation and differentiation of the vascular endothelium in a tissue-specific manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1, 27–31 (1995).
Ferrara, N. & Alitalo, K. Clinical applications of angiogenic growth factors and their inhibitors. Nature Med. 5, 1359–1364 (1999).
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 6, 389–395 (2000).
Yancopoulos, G. D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Stewart, P. A. & Wiley, M. J. Developing nervous tissue induces formation of blood–brain barrier characteristics in invading endothelial cells: a study using quail-chick transplantation chimeras. Dev. Biol. 84, 183–192 (1981).
Aird, W. C. et al. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J. Cell Biol. 138, 1117–1124 (1997).
Dellian, M., Witwer, B. P., Salehi, H. A., Yuan, F. & Jain, R. K. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am. J. Pathol. 149, 59–71 (1996).
Simionescu, N. & Simionescu, M. in Cell and Tissue Biology (ed. Weiss, L.) 355–398 (Urban & Schwarzemberg, Baltimore, 1988).
Palade, G. E., Simionescu, M. & Simionescu, N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol. Scand. (Suppl.) 463, 11–32 (1979).
Breier, G., Albrecht, U., Sterrer, S. & Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114, 521–532 (1992).
Monacci, W. T., Merrill, M. J. & Oldfield, E. H. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am. J. Physiol. 264, C995–C1002 (1993).
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Joubert, F. J. & Strydom, D. J. Snake venom. The amino acid sequence of protein A from Dendroaspis polylepis polylepis (black mamba) venom. H.-S. Z. Physiol. Chem. 361, 1787–1794 (1980).
Boisbouvier, J. et al. A structural homologue of colipase in black mamba venom revealed by NMR floating disulphide bridge analysis. J. Mol. Biol. 283, 205–219 (1998).
Wechselberger, C. et al. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 462, 177–181 (1999).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Aravind, L. & Koonin, E. V. A colipase fold in the carboxy-terminal domain of the Wnt antagonists—the Dickkopfs. Curr. Biol. 8, 477–478 (1998).
Klagsbrun, M. Mediators of angiogenesis: the biological significance of basic fibroblast growth factor (bFGF)-heparin and heparan sulfate interactions. Semin. Cancer Biol. 3, 81–87 (1992).
Zetter, B. R. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature 285, 41–43 (1980).
Arbiser, J. L. et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl Acad. Sci. USA 94, 861–866 (1997).
Roberts, W. G. & Palade, G. E. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 57, 765–772 (1997).
Esser, S. et al. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 140, 947–959 (1998).
Gospodarowicz, D., Lepine, J., Massoglia, S. & Wood, I. Comparison of the ability of basement membranes produced by corneal endothelial and mouse-derived endodermal PF-HR-9 cells to support the proliferation and differentiation of bovine kidney tubule epithelial cells in vitro. J. Cell Biol. 99, 947–961 (1984).
Semenza, G. L. HIF-1: mediator of physiological and pathophysiological response to hypoxia. J. Appl. Physiol. 88, 1474–1480 (2000).
Dor, Y., Porat, R. & Keshet, E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am. J. Physiol. 280, C1367–C1374 (2001).
Collin, O. & Bergh, A. Leydig cells secrete factors which increase vascular permeability and endothelial cell proliferation. Int. J. Androl. 19, 221–228 (1996).
Bassett, D. L. The changes in the vascular pattern of the ovary of the albino rat during the estrous cycle. Am. J. Anat. 73, 251–278 (1943).
Ravindranath, N., Little-Ihrig, L., Phillips, H. S., Ferrara, N. & Zeleznik, A. J. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology 131, 254–260 (1992).
Goldziher, J. W. & Green, J. A. The polycistic ovary. I. Clinical and histologic features. J. Clin. Endocrinol. Metab. 22, 325–332 (1962).
Phillips, H. S., Hains, J., Leung, D. W. & Ferrara, N. Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology 127, 965–967 (1990).
Pettersson, A. et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 80, 99–115 (2000).
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996).
Gerber, H. P. et al. VEGF is required for growth and survival in neonatal mice. Development 126, 1149–1159 (1999).
Ferrara, N. et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med. 4, 336–340 (1998).
Gerber, H. P. et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Med. 5, 623–628 (1999).
Kim, K. J. et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362, 841–844 (1993).
Aiello, L. P. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 331, 1480–1487 (1994).
Ferrara, N. VEGF: An update on biological and therapeutic aspects. Curr. Opin. Biotech. 11, 617–624 (2000).
Ryan, A. M. et al. Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol. Pathol. 27, 78–86 (1999).
Miao, H. Q. et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J. Cell Biol. 146, 233–242 (1999).
Schweitz, H., Pacaud, P., Diochot., Moinier, D. & Ladzunski, M. MIT(1), a black mamba intestinal toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 461, 183–188 (1999).
Phillips, H. S., Hains, J. M., Laramee, G. R., Rosenthal, A. & Winslow, J. W. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science 250, 290–294 (1990).
Melton, D. A. et al. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 12, 7035–7056 (1984).
Gille, H. et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific VEGF mutants. J. Biol. Chem. 276, 3222–3230 (2001).
Krupnik, V. E. et al. Functional and structural diversity of the human Dickkopf gene family. Gene 238, 301–313 (1999).
van Tilbeurgh, H., Sarda, L., Verger, R. & Cambillau, C. Structure of the pancreatic lipase–procolipase complex. Nature 359, 159–162 (1992).
Acknowledgements
We thank the oligonucleotide-synthesis, DNA-sequencing and protein-purification groups; D. Tran and G. Bennett for protein iodination; P. Schow for cell sorting; M. van Hoy for necropsy; and R. Taylor and P. Tobin for histology. We also thank H. Chen for initial observations and J. Singh and L. Yu for cell culture and transfections.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information
Rights and permissions
About this article
Cite this article
LeCouter, J., Kowalski, J., Foster, J. et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412, 877–884 (2001). https://doi.org/10.1038/35091000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35091000
This article is cited by
-
Prokineticin 1 is a novel factor regulating porcine corpus luteum function
Scientific Reports (2023)
-
In vivo recording of the circadian calcium rhythm in Prokineticin 2 neurons of the suprachiasmatic nucleus
Scientific Reports (2023)
-
EG-VEGF maternal levels predict spontaneous preterm birth in the second and third trimesters in pregnant women with risk factors for placenta-mediated complications
Scientific Reports (2023)
-
Biology and therapeutic targeting of vascular endothelial growth factor A
Nature Reviews Molecular Cell Biology (2023)
-
Targeting angiogenesis in oncology, ophthalmology and beyond
Nature Reviews Drug Discovery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.