Abstract
Surgical, pharmacological and genetic lesion studies have revealed distinct anatomical sites involved with different forms of learning. Studies of patients with localized brain damage and work in rodent model systems, for example, have shown that the hippocampal formation participates in acquisition of declarative tasks but is not the site of their long-term storage1,2. Such lesions are usually irreversible, however, which has limited their use for dissecting the temporal processes of acquisition, storage and retrieval of memories3,4. Studies in bees and flies have similarly revealed a distinct anatomical region of the insect brain, the mushroom body, that is involved specifically in olfactory associative learning5,6. We have used a temperature-sensitive dynamin transgene, which disrupts synaptic transmission reversibly and on the time-scale of minutes7, to investigate the temporal requirements for ongoing neural activity during memory formation. Here we show that synaptic transmission from mushroom body neurons is required during memory retrieval but not during acquisition or storage. We propose that the hebbian processes underlying olfactory associative learning reside in mushroom body dendrites or upstream of the mushroom body and that the resulting alterations in synaptic strength modulate mushroom body output during memory retrieval.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neuropsychiat. Clin. Neurosci. 12, 103–113 (2000).
Milner, B., Squire, L. R. & Kandel, E. R. Cognitive neuroscience and the study of memory. Neuron 20, 445–468 (1998).
Mayford, M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996).
Shimizu, E., Tang, Y. P., Rampon, C. & Tsien, J. Z. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science 290, 1170–1174 (2000).
de Belle, J. S. & Heisenberg, M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692–695 (1994).
Hammer, M. & Menzel, R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156 (1998).
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001).
Rybak, J. & Menzel, R. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the α-lobe. J. Comp. Neurol. 334, 444–465 (1993).
Strausfeld, N. J. & Li, Y. Organization of olfactory and multimodal afferent neurons supplying the calyx and pedunculus of the cockroach mushroom bodies. J. Comp. Neurol. 409, 603–625 (1999).
Ito, K. et al. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn. Mem. 5, 52–77 (1998).
Connolly, J. B. et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 274, 2104–2106 (1996).
Zars, T., Fischer, M., Schulz, R. & Heisenberg, M. Localization of a short-term memory in Drosophila. Science 288, 672–675 (2000).
Poodry, C. A., Hall, L. & Suzuki, D. T. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev. Biol. 32, 373–386 (1973).
Poodry, C. A. & Edgar, L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J. Cell Biol. 81, 520–527 (1979).
Ramaswami, M., Rao, S., van der Bliek, A., Kelly, R. B. & Krishnan, K. S. Genetic studies on dynamin function in Drosophila. J. Neurogenet. 9, 73–87 (1993).
Koenig, J. H. & Ikeda, K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 9, 3844–3860 (1989).
Chen, M. S. et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351, 583–586 (1991).
van der Bliek, A. M. & Meyerowitz, E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351, 411–414 (1991).
Damke, H., Baba, T., Warnock, D. E. & Schmid, S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934 (1994).
Grant, D., Unadkat, S., Katzen, A., Krishnan, K. S. & Ramaswami, M. Probable mechanisms underlying interallelic complementation and temperature-sensitivity of mutations at the shibire locus of Drosophila melanogaster. Genetics 149, 1019–1030 (1998).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Yang, M. Y., Armstrong, J. D., Vilinsky, I., Strausfeld, N. J. & Kaiser, K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron 15, 45–54 (1995).
Joiner, M. A. & Griffith, L. C. Mapping of the anatomical circuit of CaM kinase-dependent courtship conditioning in Drosophila. Learn. Mem. 6, 177–192 (1999).
Benzer, S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl Acad. Sci. USA 58, 1112–1119 (1967).
Tully, T., Preat, T., Boynton, S. C. & Del Vecchio, M. Genetic dissection of consolidated memory in Drosophila. Cell 79, 35–47 (1994).
Wang, Y. T. & Linden, D. J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25, 635–647 (2000).
Carroll, R. C. et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl Acad. Sci. USA 96, 14112–14117 (1999).
Dudai, Y., Corfas, G. & Hazvi, S. What is the possible contribution of Ca2+-stimulated adenylate cyclase to acquisition, consolidation and retention of an associative olfactory memory in Drosophila? J. Comp. Physiol. A 162, 101–109 (1988).
Waddell, S., Armstrong, J. D., Kitamoto, T., Kaiser, K. & Quinn, W. G. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103, 805–813 (2000).
Dubnau, J. & Tully, T. Functional anatomy: from molecule to memory. Curr. Biol. 11, R240–R243 (2001).
Acknowledgements
We thank H. Cline, E. Drier, J. Huang, M. Regulski and K. Svoboda for comments on the manuscript. This work was supported by the NIH (J.T.D. and T.T.) and the NSF (T.K.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
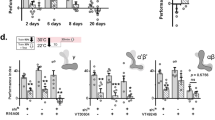
The experiments of Dubnau et al (2001) summarized in their Figure 4A, suggested that synaptic transmission in mushroom body (MB) neurons was required for retrieval – but not acquisition or storage – of olfactory memory. This interpretation was complicated by the observation that high temperature (29C) produced a general reduction in olfactory avoidance behavior in the T-maze. This nonspecific effect lowered performance indices (PIs) for control (UAS-shits1/+) and experimental (UAS-shits1/C747 and UAS-shits1/C309) genotypes by about 30 points, yielding scores near zero for the latter. Combined with data from Figure 3A, we nevertheless concluded that retrieval was defective in the experimental genotypes. The results from Figure 3A also indicated that the nonspecific effect of high temperature was apparent 30 minutes after training but not immediately after training. Hence, we repeated the retrieval experiment of Figure 4A, using a short (5 minute) retention interval. Results from that experiment (Figure 4B of Dubnau et al) still indicated a retrieval failure in the experimental genotypes, even in the absence of a nonspecific effect of high temperature on the control genotype. In addition to these experiments, we confirmed the retrieval hypothesis in novel way, using a "reversal retention" procedure.
Memory retention usually manifests as a "forgetting curve;" performance declines over time after training. Consequently, nonspecific factors (fatigue, attention, etc) that produce performance deficits cannot be distinguished from a genuine loss of memory. Reversal retention experiments attempt to dissociate memory loss from other nonspecific performance deficits by expressing memory loss as an increasing function over time. This protocol is comprised of two training sessions. During the first session, OCT is the CS+ and MCH is the CS-. A retention interval then occurs, before the second (reversal) training session, during which MCH becomes the CS+ and OCT becomes the CS-. Different groups of flies are subjected to reversal learning (training session #2) at various time-points after the first training session. Flies from all groups are tested immediately after their second training session, and a performance index (PI) is calculated in reference to the second (reversal) training session.
To understand how reversal learning influences conditioned odor avoidance responses in the T-maze, we must consider two theoretical extremes. First, reversal learning might disrupt fully memory after the first training session. If so, reversal learning would yield equivalent, maximal PIs after each reversal retention interval (see Fig. S1, fully disrupted). Second, reversal learning might not disrupt memory after the first training session at all. Instead, the reversal learning would act additively with memory after the first training session. In this case, the usual forgetting curve would manifest as an increasing function after reversal learning, and the reversal retention curve would be a mirror image of the forgetting curve (see Fig. S1, not disrupted).

Figure S1.
Reversal retention in wild type (Can-S) and amnesiac (amn) mutant flies (adapted from Tully et al. 1990) (GIF 2.21 KB)
The observed reversal retention curve of wild-type flies actually falls between these two theoretical extremes. Some memory of the first training session is disrupted by reversal learning and some is not. The stable memory appears to have two phases, an early one within the first 30 minutes and a later one that yields asymptotic performance from 30 to 180 minutes after training. Quantitatively and temporally, these stable phases are remniscent of short-term memory (STM) and anesthesia-resistant memory (ARM), while the disrupted memory might correspond to middle-term memory (MTM). The latter notion was confirmed by comparing reversal retention between wild-type flies and amnesiac mutants, the memory defect of which originally was used to define MTM. Reversal learning disrupted the same phase of memory (MTM) in wild-type flies that already was disrupted genetically in amnesiac mutants. Consequently, the reversal retention curves of wild-type and mutant flies are similar (Fig. S1).
We used this reversal retention protocol to dissociate general performance defects at restrictive temperature common to all genotypes, from a retrieval failure specific to the UAS-shits1/C747 and UAS-shits1/C309 flies. In this experiment, reversal training was always done at a 30-min retention interval, and performance was tested immediately after reversal training. If the performance defect that we observed for at the restrictive temperature in UAS-shits1/+, UAS-shits1/C747 and UAS-shits1/C309 flies (Fig. 4A) were due to a general performance decrement at the restrictive temperature all three genotypes would yield non-zero scores in this reversal learning protocol. Conversely, a retrieval failure that is specific to the UAS-shits1/C747 and UAS-shits1/C309 flies would be expected to yield PIs of zero for the UAS-shits1/C747 and UAS-shits1/C309 flies and to yield a positive score for control flies.
As shown in Figure S2, the latter is the observed outcome. At permissive temperature (20C), all three genotypes performed similarly. At restrictive temperature (29C), in contrast, the experimental genotypes scored zero, while performance of the control genotype actually increased. This genotype-specific outcome was expected only if a disruption of synaptic transmission blocks memory retrieval.

Figure S2.
30 min reversal retention reveals a retrieval-specific disruption (GIF 3.29 KB)
REFERENCES
-
1.
Tully, T., et al. Cold Spring Harbor Symp. Quant. Biol. 55, 203-211 (1990).
-
2.
Li, W., Tully, T. & Kalderon, D. Learn. Mem. 2, 320-333 (1996).
-
3.
Tully, T. in Modulation of Synaptic Transmission and Plasticity in Nervous Systems (eds. Hertting, G. & Spatz, H.-C.) 401-417 (Springer-Verlag, Berlin, 1988).
-
4.
Dubnau, J. & Tully, T. Ann. Rev. Neurosci. 21, 407-444 (1998)
Rights and permissions
About this article
Cite this article
Dubnau, J., Grady, L., Kitamoto, T. et al. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 411, 476–480 (2001). https://doi.org/10.1038/35078077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35078077
This article is cited by
-
Multisensory learning binds neurons into a cross-modal memory engram
Nature (2023)
-
Visualization of learning-induced synaptic plasticity in output neurons of the Drosophila mushroom body γ-lobe
Scientific Reports (2022)
-
Structural aspects of plasticity in the nervous system of Drosophila
Neural Development (2018)
-
Long-term memory requires sequential protein synthesis in three subsets of mushroom body output neurons in Drosophila
Scientific Reports (2017)
-
The differential requirement of mushroom body α/β subdivisions in long-term memory retrieval in Drosophila
Protein & Cell (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.