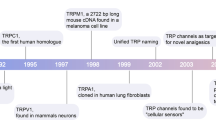
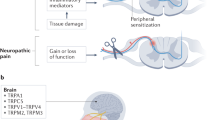
Key Points
-
Transient receptor potential (TRP) channels are tetramers assembled from subunits with six membrane-spanning domains, are permeable to monovalent cations and calcium, and are linked to the phosphatidylinositol signal transduction pathway. There are at least 20 members in the TRP family, but their function remains largely unknown.
-
The TRP family can be divided by sequence homology into three subfamilies — short, long and osm-9-like TRP channels — which are referred to in this review as TRPCx (short), TRPVx (osm-9-like or vanilloid) and TRPMx (long or melastatin). The most distinctive segments among the three subfamilies are located along the carboxy terminal, whereas the most conserved regions are in the S6 domain, which is presumably involved in gating.
-
The TRPC group can be divided into four subfamilies (TRPC1, TRPC4,5, TRPC3,6,7 and TRPC2) on the basis of sequence homology and functional similarities. This group might include the receptor-operated calcium channels long recognized by physiologists.
-
The TRPV group has five members — TRPV1–5. TRPV1 is the best-characterized member of the group; it is responsive to heat, pH and capsaicin, and is thought to participate in pain processing. TRPV6 is a possible molecular correlate of the long-known calcium-release-activated current ICRAC, but the evidence to support this idea is still incomplete.
-
The TRPM subfamily has eight members divided into four groups. The TRPM family has a potential role in cell-cycle regulation, as the expression of TRPM1 has been related to skin cancer. Another intriguing member of the family is TRPM7, the first example of a channel with kinase activity, which can actually modulate channel function.
Abstract
Mammalian homologues of the Drosophila transient receptor potential (TRP) channel gene encode a family of at least 20 ion channel proteins. They are widely distributed in mammalian tissues, but their specific physiological functions are largely unknown. A common theme that links the TRP channels is their activation or modulation by phosphatidylinositol signal transduction pathways. The channel subunits have six transmembrane domains that most probably assemble into tetramers to form non-selective cationic channels, which allow for the influx of calcium ions into cells. Three subgroups comprise the TRP channel family; the best understood of these mediates responses to painful stimuli. Other proposed functions include repletion of intracellular calcium stores, receptor-mediated excitation and modulation of the cell cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harteneck, C., Plant, T. D. & Schultz, G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 23, 159–166 (2000).The first comprehensive review of TRP channels from many species. It contains the most widely used nomenclature of the members of this family. The original contributions by Montell, Rubin, Hardie, Minke and co-workers in Drosophila are detailed.
Clapham, D. E. Calcium signaling. Cell 80, 259–268 (1995).
Hardie, R. C. & Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651 (1992).
Acharya, J. K., Jalink, K., Hardy, R. W., Hartenstein, V. & Zuker, C. S. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron 18, 881–887 (1997).With reference 5 , provides evidence against the InsP 3 R conformational-coupling hypothesis (direct TRP channel gating by the InsP 3 R).
Raghu, P. et al. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol. Cell. Neurosci. 15, 429–445 (2000).
Hardie, R. C. et al. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30, 1–20 (2001).
Rebecchi, M. J. & Pentyala, S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 (2000).
Rhee, S. G. & Bae, Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272, 15045–15048 (1997).
Berridge, M., Lipp, P. & Bootman, M. Calcium signalling. Curr. Biol. 9, R157–R159 (1999).
Kim, Y. H. et al. Phospholipase Cδ1 is activated by capacitative calcium entry that follows phospholipase Cβ activation upon bradykinin stimulation. J. Biol. Chem. 274, 26127–26134 (1999).
Putney, J. W. Jr Muscarinic, α-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J. Physiol. 268, 139–149 (1977).The first paper describing the store-depletion hypothesis (capacitative coupling).
Lewis, R. S. & Cahalan, M. D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1, 99–112 (1989).Lewis and Cahalan described a small highly selective Ca2+ conductance in lymphocytes that was activated by low intracellular calcium. This current was subsequently named I CRAC by Hoth and Penner (reference 13).
Hoth, M. & Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 (1992).Hoth and Penner correlated the small calcium-selective conductance in blood cells with depletion of calcium stores and named it I CRAC , for Ca2+ release activated current.
Zitt, C. et al. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16, 1189–1196 (1996).
Lintschinger, B. et al. Coassembly of trp1 and trp3 proteins generates diacylglycerol- and Ca2+- sensitive cation channels. J. Biol. Chem. 275, 27799–27805 (2000).
Strubing, C., Krapivinsky, G., Krapivinsky, L. & Clapham, D. E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29, 645–655 (2001).Showed that TRPC channels formed heteromers in mammalian brain, which correlated with native currents from the hippocampus and cortex. The TRPC1/TRPC5 and TRPC1/TRPC4 heteromers were activated by G q -linked receptors, but not by store depletion.
Okada, T. et al. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem. 273, 10279–10287 (1998).
Schaefer, M. et al. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 275, 17517–17526 (2000).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Tang, Y. et al. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J. Biol. Chem. 275, 37559–37564 (2000).
Freichel, M. et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nature Cell Biol. 3, 121–127 (2001).This work revealed a physiological function of TRPC4 in the regulation of vascular tone in mice, and provided the strongest evidence so far for a store-dependent activation of mammalian TRP homologues. The authors noted a decrease in a putative endothelial I CRAC current and concluded that TRPC4 formed part of the I CRAC channel. However, intriguing questions remain since TRPC4 channels are themselves not Ca2+-selective.
Philipp, S. et al. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 17, 4274–4282 (1998).
Zitt, C. et al. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J. Cell Biol. 138, 1333–1341 (1997).Presented the first unequivocal evidence that TRPCs form functional ion channels and challenged the concept of TRPCs being store-operated by showing that TRPC3 is activated by intracellular Ca2+ but not by store depletion.
Li, H. S., Xu, X. Z. & Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24, 261–273 (1999).
Estacion, M., Sinkins, W. G. & Schilling, W. P. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 530, 1–19 (2001).
Kiselyov, K. et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 396, 478–482 (1998).
Okada, T. et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 274, 27359–27370 (1999).
Inoue, R. et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 88, 325–332 (2001).
Liman, E. R., Corey, D. P. & Dulac, C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl Acad. Sci. USA 96, 5791–5796 (1999).
Vannier, B. et al. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc. Natl Acad. Sci. USA 96, 2060–2064 (1999).
Wes, P. D. et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl Acad. Sci. USA 92, 9652–9656 (1995).
Hofmann, T., Schaefer, M., Schultz, G. & Gudermann, T. Cloning, expression and subcellular localization of two novel splice variants of mouse transient receptor potential channel 2. Biochem. J. 351, 115–122 (2000).
Jungnickel, M. K. et al. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biol. 3, 499–502 (2001).
Fukami, K. et al. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science 292, 920–923 (2001).
Caterina, M. J. & Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517 (2001).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).The initial identification of the capsaicin-receptor channel, founding member of the TRPV subfamily. This work led to the identification of VR-1 and VRL-1 channels, and their role in pain pathways.
Caterina, M. J., Rosen, T. A., Tominaga, M., Brake, A. J. & Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441 (1999).
Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G. & Plant, T. D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biol. 2, 695–702 (2000).
Liedtke, W. et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 (2000).
Hoenderop, J. G. et al. Molecular identification of the apical Ca2+ channel in 1,25 dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 274, 8375–8378 (1999).
Vennekens, R. et al. Permeation and gating properties of the novel epithelial Ca2+ channel. J. Biol. Chem. 275, 3963–3969 (2000).
Nilius, B. et al. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J. Physiol. 527, 239–248 (2000).
Tsien, R. W., Hess, P., McCleskey, E. W. & Rosenberg, R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu. Rev. Biophys. Biophys. Chem. 16, 265–290 (1987).
Nilius, B. et al. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J. Biol. Chem. 276, 1020–1025 (2001).
Peng, J. B. et al. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 274, 22739–22746 (1999).
Yue, L., Peng, J. B., Hediger, M. A. & Clapham, D. E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410, 705–709 (2001).Showed that CaT1, like ECaC (see references 40 – 42 ), is highly Ca2+-selective and exhibits the biophysical properties of the putative I CRAC current identified by Cahalan and co-workers under divalent-free conditions.
Runnels, L. W., Yue, L. & Clapham, D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001).Characterizes the ion channel and kinase properties of the novel protein TRP-PLIK, showing that the kinase activity of the protein is required for proper channel function. It is also the first electrophysiological study of a member of the TRPM subgroup.
Nagamine, K. et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54, 124–131 (1998).
Perraud, A.-L. et al. ADP-ribose gating of the calcium-permeable LTRP62 channel revealed by Nudix motif homology. Nature (in the press).
Bessman, M. J., Frick, D. N. & O'Handley, S. F. The MutT proteins or 'Nudix' hydrolases, a family of versatile, widely distributed, 'housecleaning' enzymes. J. Biol. Chem. 271, 25059–25062 (1996).
Tsavaler, L., Shapero, M. H., Morkowski, S. & Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769 (2001).
Duncan, L. M. et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 58, 1515–1520 (1998).
Hunter, J. J. et al. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 54, 116–123 (1998).
Fang, D. & Setaluri, V. Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem. Biophys. Res. Commun. 279, 53–61 (2000).
Deeds, J., Cronin, F. & Duncan, L. M. Patterns of melastatin mRNA expression in melanocytic tumors. Hum. Pathol. 31, 1346–1356 (2000).
Duncan, L. M. et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J. Clin. Oncol. 19, 568–576 (2001).This clinical study validates that expression of the melastatin gene assists with the prognosis of melanoma. It is also the first report linking TRP channels to a specific pathology.
Prawitt, D. et al. Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum. Mol. Genet. 9, 203–216 (2000).
Zhu, X. et al. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 85, 661–671 (1996).Initial identification (see also reference 31 ) of several members of the TRPC channel, arguing for the involvement of TRPC1 and TRPC3 in capacitative Ca2+ entry by expression of antisense cDNAs.
Kamouchi, M. et al. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J. Physiol. 518, 345–358 (1999).
Ohki, G. et al. A calcium-activated cation current by an alternatively spliced form of Trp3 in the heart. J. Biol. Chem. 275, 39055–39060 (2000).
Philipp, S. et al. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 15, 6166–6171 (1996).
Mori, Y. et al. Differential distribution of TRP Ca2+ channel isoforms in mouse brain. Neuroreport 9, 507–515 (1998).
Boulay, G. et al. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 272, 29672–29680 (1997).
Walker, R. L., Hume, J. R. & Horowitz, B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am. J. Physiol. Cell Physiol. 280, C1184–C1192 (2001).
Premkumar, L. S. & Ahern, G. P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990 (2000).
Kanzaki, M. et al. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nature Cell Biol. 1, 165–170 (1999).
Peng, J. B. et al. A rat kidney-specific calcium transporter in the distal nephron. J. Biol. Chem. 275, 28186–28194 (2000).
Peng, J. B. et al. Human calcium transport protein CaT1. Biochem. Biophys. Res. Commun. 278, 326–332 (2000).
Enklaar, T. et al. Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics 67, 179–187 (2000).
Saitou, N. & Nei, M. The neighbor–joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
Glossary
- POLYCYSTIC KIDNEY DISEASE PROTEINS
-
Also known as polycystins, these proteins form cation-selective channels that are permeable to calcium. Mutations in the polycystins lead to the formation of fluid-filled cysts in the kidneys.
- ANKYRINS
-
Adapter molecules that couple membrane proteins to the spectrin-based membrane cytoskeleton.
- PLECKSTRIN-HOMOLOGY DOMAINS
-
Sequences of about 100 amino acids present in many signalling molecules. Pleckstrin is a protein of unknown function originally identified in platelets. It is a principal substrate of protein kinase C.
- EF-HANDS
-
Ca2+-binding domains originally identified in parvalbumin, which are also known as helix–turn–helix domains.
- SH DOMAINS
-
Src-homology domains. They are involved in the interaction with phosphorylated tyrosine residues on other proteins (SH2 domains) or with proline-rich sections of other proteins (SH3 domains).
- PERTUSSIS TOXIN
-
The causative agent of whooping cough, pertussis toxin causes the persistent activation of Gi proteins by catalysing the ADP-ribosylation of the α-subunit.
- PDZ DOMAIN
-
A peptide-binding domain that is important for the organization of membrane proteins, particularly at cell–cell junctions, including synapses. They can bind to the carboxy termini of proteins, or can form dimers with other PDZ domains. PDZ domains are named after the proteins in which these sequence motifs were originally identified (PSD95, Discs-large, zona occludens-1).
- TRANSDUCISOME
-
Signalling complex in the Drosophila eye that is formed by the components of the phototransduction cascade, including the ion channels, the phospholipase Cβ and protein kinase C.
- RECTIFICATION
-
The property whereby current through a channel does not flow with the same ease from the inside as from the outside. In inward rectification, for instance, current into the cell flows more easily than out of the cell through the same population of channels.
- PSEUDOGENE
-
A sequence in DNA that is related to a functional gene but cannot be transcribed due to mutational changes or the lack of regulatory sequences.
- THAPSIGARGIN
-
Plant diterpene that blocks smooth endoplasmic Ca2+–ATPase pumps and depletes Ca2+ stores.
- CURRENT–VOLTAGE RELATIONSHIP
-
A plot of the changes in ionic current as a function of membrane voltage.
- ANOMALOUS MOLE-FRACTION BEHAVIOUR
-
When two or more ions simultaneously reside inside a channel, their movement through the pore is dependent on each other. When channel conductance is measured as a function of the concentration ratio of two different ionic species and the conductance goes through a minimum rather than changing linearly as the ratio changes, then it is said to show anomalous mole-fraction behaviour.
- BECKWITH–WIEDEMANN SYNDROME
-
Syndrome of unknown aetiology characterized by the presence of macroglossia (large tongue), visceromegaly (large organs), macrosomia (large body size) and hypoglycemia. Patients show an increased susceptibility to tumour development.
Rights and permissions
About this article
Cite this article
Clapham, D., Runnels, L. & Strübing, C. The trp ion channel family . Nat Rev Neurosci 2, 387–396 (2001). https://doi.org/10.1038/35077544
Issue Date:
DOI: https://doi.org/10.1038/35077544
This article is cited by
-
Future targets for migraine treatment beyond CGRP
The Journal of Headache and Pain (2023)
-
Radiosynthesis and Evaluation of a C-11 Radiotracer for Transient Receptor Potential Canonical 5 in the Brain
Molecular Imaging and Biology (2023)
-
Therapeutic Potential of Combinative shRNA-Encoded Lentivirus-Mediated Gene Silencing to Accelerate Somatosensory Recovery After Spinal Cord Trauma
Neurotherapeutics (2023)
-
Neural Mechanisms Underlying the Coughing Reflex
Neuroscience Bulletin (2023)
-
The industrial solvent 1,4-dioxane causes hyperalgesia by targeting capsaicin receptor TRPV1
BMC Biology (2022)