Key Points
-
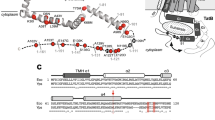
The twin-arginine translocase (Tat) system operates in the chloroplast thylakoid membrane and the plasma membranes of most bacteria.
-
It recognizes substrates that bear signal peptides containing a twin-arginine motif before a hydrophobic region.
-
It is driven, at least in thylakoids, by the proton gradient across the membrane; nucleoside triphosphate hydrolysis is not required.
-
The Tat system is, at present, unique in that it can transport fully folded proteins across tightly sealed membranes.
-
Its substrates include globular proteins that must bind certain cofactors before transport, as well as proteins that seem to fold too quickly/tightly for 'conventional' Sec-type systems to handle.
-
Three genes have been shown to encode important Tat components in Escherichia coli, namely tatA, tatB and tatC.
-
The isolated Tat complex is about 600 kDa but has yet to be analysed in an active state.
Abstract
The twin-arginine translocation pathway operates in the thylakoid membrane of chloroplasts and in the plasma membrane of most free-living bacteria. Its main function is to transport fully folded proteins across the membrane. Three important tat genes have been identified and the sequences of the encoded proteins, together with the unusual properties of the pathway, indicate that the Tat system is completely different from other protein translocases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Blobel, G. & Dobberstein, B. Transfer of proteins across membranes. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851 ( 1975).
Jungnickel, B., Rapoport, T. & Hartmann, E. Protein translocation: common themes from bacteria to man. FEBS Lett. 346, 73– 77 (1994).
Schatz, G. & Dobberstein, B. Common principles of protein translocation across membranes. Science 271, 1519–1526 (1996).
Izard, J. W. & Kendall, D. A. Signal peptides: exquisitely designed transport promoters. Mol. Microbiol. 13, 765–773 (1994).
Dalbey, R. E. & Robinson, C. Protein translocation into and across the bacterial plasma membrane and the plant thylakoid membrane. Trends Biochem. Sci. 24, 17–22 (1999).
Heins, L., Collinson, I. & Soll, J. The protein transport apparatus of chloroplast envelopes . Trends Plant Sci. 3, 56– 61 (1998).
Herrmann, J. M. & Neupert, W. Protein transport into mitochondria. Curr. Opin. Microbiol. 3, 210–214 (2000).
Yuan, J., Henry, R., McCaffery, M. & Cline, K. SecA homolog in protein transport within chloroplasts: evidence for endosymbiont-derived sorting . Science 266, 796–798 (1994).
Laidler, V., Chaddock, A. M., Knott, T. G., Walker, D. & Robinson, C. A SecY homolog in Arabidopsis thaliana. Sequence of a full-length cDNA clone and import of the precursor protein into chloroplasts. J. Biol. Chem. 270, 17664–17667 (1995).
Schuenemann, D., Amin, P., Hartmann, E. & Hoffman, N. E. Chloroplast SecY is complexed to SecE and involved in the translocation of the 33-kDa, but not the 23-kDa subunit of the oxygen-evolving complex. J. Biol. Chem. 274, 12177–12182 ( 1999).
Mould, R. M. & Robinson, C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J. Biol. Chem. 266, 12189– 12193 (1991).
Line, K., Ettinger, W. F. & Theg, S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem. 267, 2688–2696 (1992).
Klösgen, R. B., Brock, I. W., Herrmann, R. G. & Robinson, C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol. Biol. 18, 1031–1034 ( 1992).
Robinson, C. et al. The presequence of a chimeric construct dictates which of two mechanisms are utilized for translocation across the thylakoid membrane: evidence for the existence of two distinct translocation systems. EMBO J. 13, 279–285 ( 1994).
Henry, R., Kapazoglou, A., McCaffery, M. & Cline, K. Differences between lumen targeting domains of chloroplast transit peptides determine pathway specificity for thylakoid transport. J. Biol. Chem. 269, 10189–10192 ( 1994).
Chaddock, A. M. et al. A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the pH-dependent thylakoidal protein translocase . EMBO J. 14, 2715–2722 (1995).This paper showed for the first time the central importance of the twin-arginine motif in the Tat pathway.
Voelker, R. & Barkan, A. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 14, 3905–3914 ( 1995).
Settles, M. A. et al. Sec-independent protein translocation by the maize Hcf106 protein. Science 278, 1467– 1470 (1997).This was the first report of the hcf106 sequence and the first indication that the system might be present in bacteria.
Weiner, J. H. et al. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93, 93–101 (1998).The first report of a tat mutant, although the affected gene was incorrectly sequenced and believed to encompass both tatA and tatB.
Sargent, F. et al. Overlapping functions of components of a bacterial Sec-independent export pathway. EMBO J. 17, 3640– 3650 (1998).The first description of the detailed phenotypes of tatA and tatE disruptions.
Sargent, F., Stanley, N. R., Berks, B. C. & Palmer, T. Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J. Biol. Chem. 274, 36073–36082 (1999).
Bogsch, E. G. et al. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273, 18003–18006 ( 1998).First demonstration of the central importance of TatC.
Wexler, M. et al. TatD is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in Sec-independent protein export. J. Biol. Chem. 275, 16717–16722 (2000).
Mori, H., Summer, E. J., Ma, X. & Cline, K. Component specificity for the thylakoidal Sec and delta pH-dependent protein transport pathways . J. Cell Biol. 146, 45– 55 (1999).
Walker, M. B., Roy, L. M., Coleman, E., Voelker, R. & Barkan, A. The maize tha4 gene functions in Sec-independent protein transport in chloroplasts and is related to hcf106, tatA and tatB. J. Cell Biol. 147, 267–275 (1999).
Clark, S. A. & Theg, S. M. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell 8, 923–934 ( 1997).
Hynds, P. J., Robinson, D. & Robinson, C. The Sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273, 34868– 34874 (1998).References 26 and 27 showed for the first time that the thylakoid Tat system can transport folded proteins.
Thomas, J. D., Daniel, R. A., Errington, J. & Robinson, C. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39, 47–52 ( 2001).
Santini, C.-L. et al. Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of its periplasmic localization in response to osmotic up–shock. J. Biol. Chem. 276, 8159–8164 (2001).
Feilmeier, B. J., Iseminger, G., Schroeder, D., Webber, H. & Phillips, G. J. Green fluorescent protein functions as a reporter for protein localisation in Escherichia coli. J. Bacteriol. 182, 4068–4076 (2000).
Berks, B. C. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol 22, 393–404 (1996).This is a comprehensive description of the rationale for the existence of the Tat pathway and a listing of predicted substrates.
Santini, C. L. et al. A novel Sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17, 101–112 (1998).
Rodrigue, A., Chanal, A., Beck, K., Müller, M. & Wu, L.-F. Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial Tat-pathway. J. Biol. Chem. 274, 13223–13228 (1999).
Koussevitzky, S., Ne'eman, E., Sommer, A., Steffens, J. C. & Harel, E. Purification and properties of a novel chloroplast stromal peptidase. Processing of polyphenol oxidase and other imported precursors . J. Biol. Chem. 273, 27064– 27069 (1998).
Henry, R., Carrigan, M., McCaffrey, M., Ma, X. & Cline, K. Targeting determinants and proposed evolutionary basis for the Sec and the delta pH protein transport systems in chloroplast thylakoid membranes. J. Cell Biol. 136 , 823–832 (1997).
Bogsch, E., Brink, S. & Robinson, C. Pathway specificity for a pH-dependent precursor thylakoid lumen protein is governed by a 'Sec-avoidance' motif in the transfer peptide and a 'Sec-incompatible' mature protein. EMBO J. 16 , 3851–3858 (1997).
Simonen, M. & Palva, I. Protein secretion in Bacillus species. Microbiol. Rev. 57, 109– 137 (1993).
Hirose, I. et al. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146, 65–75 ( 2000).
Jongbloed, J. D. H. et al. TatC is a specificity determinant for protein secretion by the twin-arginine translocation pathway. J. Biol. Chem. 275, 41350–41357 (2000).
Cristobal, S., de Gier, J. W., Nielsen, H. & von Heijne, G. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18, 2982– 2990 (1999).Suggests that hydrophobicity influences to a large extent the maintenance of the Sec/Tat pathway specificity in E. coli.
Bolhuis, A., Bogsch, E. G. & Robinson, C. Subunit interactions in the twin-arginine translocase complex of Escherichia coli. FEBS Lett. 472, 88–92 (2000).
Bolhuis, A., Mathers, J. E., Thomas, J. D., Barrett, C. M. L. & Robinson, C. TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli . J. Biol. Chem. (in the press).
Wu, L.-F., Ize, B., Chanal, A., Quentin, Y. & Fichant, G. Bacterial twin-arginine signal peptide-dependent protein translocation pathway: evolution and mechanism. J. Mol. Microbiol. Biotechnol. 2, 179–189 ( 2000).
Teter, S. A. & Theg, S. M. Energy-transducing thylakoid membranes remain highly impermeable to ions during protein translocation. Proc. Natl Acad. Sci. USA 95, 1590– 1594 (1998).
Hynds, P. J., Plücken, H., Westhoff, P. & Robinson, C. Different lumen-targeting pathways for nuclear-encoded versus cyanobacterial/plastid-encoded Hcf136 proteins. FEBS Lett. 467, 97– 100 (2000).
Brink, S., Bogsch, E. G., Edwards, W. R., Hynds, P. J. & Robinson, C. Targeting of thylakoid proteins by the ΔpH-driven twin-arginine translocation pathway requires a specific signal in the hydrophobic domain in conjunction with the twin-arginine motif . FEBS Lett. 434, 425–430 (1998).
Mori, H. & Cline, K. A signal peptide that directs non-Sec transport in bacteria also directs efficient and exclusive transport on the thylakoid delta pH pathway. J. Biol. Chem. 273, 11405–11408 (1998).
Wexler, M., Bogsch, E. G., Palmer, T., Robinson, C. & Berks, B. C. Targeting signals for a bacterial Sec-independent export system direct plant thylakoid import by the ΔpH pathway. FEBS Lett. 431, 339– 342 (1998).
Halbig, D., Hou, B., Freudl, R., Sprenger, G. A. & Klösgen, R. B. Bacterial proteins carrying twin-R signal peptides are specifically targeted by the ΔpH-dependent transport machinery of the thylakoid membrane system. FEBS Lett. 447, 95–98 (1999).
Stanley, N. R., Palmer, T. & Berks, B. C. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275, 11591–11596 (2000).
Author information
Authors and Affiliations
Supplementary information
Related links
Related links
DATABASE LINKS
FURTHER INFORMATION
Department of Biological Sciences at Warwick University
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- THYLAKOID
-
Sac-like vesicle that contains the photosynthetic pigments in photosynthetic organisms.
- OXYGEN-EVOLVING COMPLEX
-
Extrinsic group of photosystem II proteins, bound to the lumenal face of the thylakoid membrane.
- OPERON
-
A unit of genes in prokaryotes that is expressed as a single messenger RNA.
- MONOCISTRONIC
-
Encoding a single polypeptide or protein.
- PERIPLASM
-
Area between the inner and outer membranes of Gram-negative bacteria.
- DIHYDROFOLATE REDUCTASE
-
Enzyme that catalyses the reduction of different forms of hydrofolate. These reduction reactions are linked to the reduction/oxidation of NAD+/NADH.
- GREEN FLUORESCENT PROTEIN
-
(GFP). Autofluorescent protein, originally identified in the jellyfish Aequorea victoria.
- POLYPHENOL OXIDASE
-
Family of copper-containing oxidoreductases that catalyse the oxidation of mono- and o-diphenols to o-diquinones. Found in plants, located in the membranes of plastids (cytoplasmic organelles bound by a double membrane).
- PLASTOCYANIN
-
Soluble protein found in eukaryotic plants, which contains one atom of copper and is blue when oxidized.
- LEADER PEPTIDASE
-
Enzyme that catalyses the cleavage of the amino-terminal leader sequence from a secreted protein.
- GRAM-NEGATIVE BACTERIA
-
Bacteria whose cell walls do not retain a basic dye during the Gram-stain procedure. These cell walls are thin, consisting of a layer of lipopolysaccharide outside a peptidoglycan layer.
- VESICLE TRANSPORT
-
Transport process during which cargo-containing vesicles bud off from one membrane and are transported to another membrane with which they fuse. This allows lumenal molecules to move from one organelle to the other without ever being in contact with the cytosol.
- EUBACTERIA
-
One of the three main lineages of cellular organism, the other two being Archae and eukaryotes.
- BIP
-
Molecular chaperone in the endoplasmic reticulum that assists protein folding after import.
- MITOCHONDRIAL MATRIX
-
Space enclosed by the mitochondrial inner membrane, which contains enzymes that mediate fatty-acid oxidation and the tricarboxylic-acid cycle.
- HSP70
-
Family of heat-shock proteins, 70 kDa in size. Heat-shock proteins are molecular chaperones that are synthesized by cells that have been exposed to a temperature that is higher than normal.
- CHAPERONE
-
A protein that facilitates the proper folding of other proteins but does not bind to their final folded form.
- PROTON MOTIVE FORCE
-
Proton electrochemical gradient established by energy-transducing membranes.
- FLAGELLAR MOTION
-
Movement of bacteria and some unicellular eukaryotes that is generated using a specialized appendage termed the flagellum.
- ATP SYNTHASE
-
Enzyme that catalyses the phosphorylation of ADP to ATP during oxidative phosphorylation in mitochondria or photophosphorylation in photosynthetic organisms.
Rights and permissions
About this article
Cite this article
Robinson, C., Bolhuis, A. Protein targeting by the twin-arginine translocation pathway. Nat Rev Mol Cell Biol 2, 350–356 (2001). https://doi.org/10.1038/35073038
Issue Date:
DOI: https://doi.org/10.1038/35073038
This article is cited by
-
The ten amino acids of the oxygen-evolving enhancer of tobacco is sufficient as the peptide residues for protein transport to the chloroplast thylakoid
Plant Molecular Biology (2021)
-
The DnaJ-like zinc finger domain protein ORANGE localizes to the nucleus in etiolated cotyledons of Arabidopsis thaliana
Protoplasma (2016)
-
Signal peptide of cellulase
Applied Microbiology and Biotechnology (2014)
-
Denitrification by plant roots? New aspects of plant plasma membrane-bound nitrate reductase
Protoplasma (2012)
-
Modularity analysis based on predicted protein-protein interactions provides new insights into pathogenicity and cellular process of Escherichia coli O157:H7
Theoretical Biology and Medical Modelling (2011)