Abstract
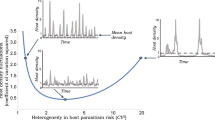
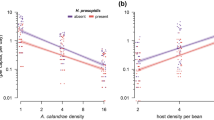
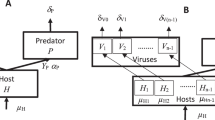
Although individual species persist within a web of interactions with other species, data are usually gathered only from the focal species itself. We ask whether evidence of a species’ interactions be detected and understood from patterns in the dynamics of that species alone. Theory predicts that strong coupling between a prey and a specialist predator/parasite should lead to an increase in the dimensionality of the prey's dynamics, whereas weak coupling should not. Here we describe a rare test of this prediction. Two natural enemies were added separately to replicate populations of a moth. For biological reasons that we identify here, the prediction of increased dimensionality was confirmed when a parasitoid wasp was added (although this increase had subtleties not previously appreciated), but the prediction failed for an added virus. Thus, an imprint of the interactions may be discerned within time-series data from component species of a system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hassell, M. P. & May, R. M. Generalist and specialist natural enemies in insect predator–prey interactions. J. Anim. Ecol. 55, 923–940 (1986).
Begon, M., Sait, S. M. & Thompson, D. J. Predator–prey cycles with period shifts between two- and three-species systems. Nature 381, 311–315 (1996).
Murdoch, W. W. & Stewart-Oaten, A. Predation and population stability. Adv. Ecol. Res. 9, 1–131 (1975).
Royama, T. Analytical Population Dynamics (Chapman & Hall, London, 1992).
Turchin, P. Rarity of density dependence or population regulation with lags? Nature 344, 660–663 (1990).
Stenseth, N. C., Falck, W., Bjørnstad, O. N. & Krebs, C. J. Population regulation in snowshoe hare and lynx populations: asymmetric food web configurations between the snowshoe hare and the lynx. Proc. Natl Acad. Sci. USA 94, 5147–5152 (1997).
Schaffer, W. M. Ecological abstraction: The consequence of reduced dimensionality in ecological models. Ecol. Monogr. 51, 383–401 (1981).
Berryman, A. A. Population Systems: a General Introduction (Plenum, New York, 1981).
Sait, S. M., Begon, M. & Thompson, D. J. Long-term population dynamics of the Indian meal worm moth Plodia interpunctella and its granulosis virus. J. Anim. Ecol. 63, 861–870 (1994).
Sait, S. M., Begon, M. & Thompson, D. J. The influence of larval age on the response of Plodia interpunctella to a granulosis virus. J. Invert. Pathol. 63, 107–110 (1994).
Sait, S. M., Begon, M. & Thompson, D. J. The effect of a sublethal baculovirus infection in the Indian meal moth, Plodia interpunctella. J. Anim. Ecol. 63, 541–550 (1994).
Gurney, W. S. C., Nisbet, R. M. & Lawton, J. H. The systematic formulation of tractable single-species population models incorporating age structure. J. Anim. Ecol. 52, 479–495 (1983).
Gurney, W. S. C. & Nisbet, R. M. Fluctuation periodicity, generation separation, and the expression of larval competition. Theor. Popul. Biol. 28, 150–180 (1985).
Briggs, C. J., Sait, S. M., Begon, M., Thompson, D. J. & Godfray, H. C. J. What causes generation cycles in populations of stored product moths? J. Anim. Ecol. 69, 352–366 (2000).
Bjørnstad, O. N. et al. Population dynamics of the Indian meal moth: demographic stochasticity and delayed regulatory mechanisms. J. Anim. Ecol. 67, 110–126 (1998).
Sait, S. M. et al. Venturia canescens parasitizing Plodia interpunctella: host vulnerability – a matter of degree. Ecol. Entomol. 20, 199–201 (1995).
Sait, S. M., Begon, M., Thompson, D. J., Harvey, J. A. & Hails, R. S. Factors affecting host selection in an insect host–parasitoid interaction. Ecol. Entomol. 22, 225–230 (1997).
Harvey, J. A., Harvey, I. F. & Thompson, D. J. Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology 75, 1420–1428 (1994).
Boots, M. Cannibalism and the stage-dependent transmission of a viral pathogen of the Indian meal moth, Plodia interpunctella. Ecol. Entomol. 23, 118–122 (1998).
Knell, R. J., Begon, M. & Thompson, D. J. Transmission of Plodia interpunctella granulosis virus does not conform to the mass action model. J. Anim. Ecol. 67, 592–599 (1998).
Cheng, B. & Tong, H. On consistent nonparametric order determination and chaos. J. R. Statist. Soc. B 54, 427–449 (1992).
Cheng, B. & Tong, H. Orthogonal projection, embedding dimension and sample size in chaotic time series from a statistical perspective. Phil. Trans. R. Soc. Lond. A 348, 325–341 (1994).
Yao, Q. & Tong, H. Quantifying the influence of initial values on non-linear prediction. J. R. Statist. Soc. B 56, 701-725 (1994).
Ellner, S. P. et al. Noise and nonlinearity in measles epidemics: Combining mechanistic and statistical approaches to population modeling. Am. Nat. 151, 425–440 (1998).
Bjørnstad, O. N., Fromentin, J.-M., Stenseth, N. C. & Gjøsaeter, J. A new test for density-dependent survival: the case of coastal cod populations. Ecology 80, 1278–1288 (1999).
Free, C. A., Beddington, J. R. & Lawton, J. H. Inadequacy of simple-models of mutual interference for parasitism and predation. J. Anim. Ecol. 46, 543–554 (1977).
Sokal, R. R. & Rohlf, F. J. in Biometry 1 Ch. XIX, 887 (W. H. Freeman, New York, 1995).
Murdoch, W. W. & Briggs, C. J. Theory of biological control: recent developments. Ecology 77, 2001–2013 (1996).
Tabashnik, B. E. & McGaughey, W. H. Resistance risk assessment for single and multiple insecticides: Responses of Indian meal moth (Lepidoptera: Pyralidae) to Bacillus thuringiensis. J. Econ. Entomol. 87, 835–841 (1994).
Costantino, R. F., Desharnais, R. A., Cushing, J. M. & Dennis, B. Chaotic dynamics in an insect population. Science 275, 389–391 (1997).
Bjørnstad, O. N., Fromentin, J.-M., Stenseth, N. C. & Gjøsaeter, J. Cycles and trends in cod population. Proc. Natl Acad. Sci. USA 96, 5066–5071 (1999).
Grenfell, B. T. & Dobson, A. P. Ecology of infectious diseases in natural populations. (Cambridge Univ. Press, Cambridge, 1995).
Nicholson, A. J. & Bailey, V. A. The balance of animal populations. Proc. Zool. Soc. Lond. 3, 551–598 (1935).
Venables, W. N. & Ripley, B. D. in Modern Applied Statistics with S-plus 1–462 (Springer, New York, 1994).
Wei, W. W. Time Series Analysis (Addison Wesley, California, 1989).
Green, P. J. & Silverman, B. W. in Nonparametric Regression and Generalized Linear Models: a Roughness Penalty Approach Ch. 1-IX, 182 (Chapman & Hall, London, 1994).
Ellner, S. & Turchin, P. Chaos in a noisy world: New methods and evidence from time-series analysis. Am. Nat. 145, 343–375 (1995).
Ricker, W. E. Stock and recruitment. J. Fish. Res. Board Canada 11, 559–623 (1954).
Acknowledgements
Funding was received from the National Center for Ecological Analysis and Synthesis (O.N.B.) (a Center funded by the NSF, the University of California Santa Barbara and the State of California), from the Norwegian National Science Foundation (O.N.B., N.C.S.) and from NERC (M.B., S.M.S. and D.J.T.). P. Amarasekare, A. Dobson and B. Grenfell commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjørnstad, O., Sait, S., Stenseth, N. et al. The impact of specialized enemies on the dimensionality of host dynamics. Nature 409, 1001–1006 (2001). https://doi.org/10.1038/35059003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35059003
This article is cited by
-
Predation and fragmentation portrayed in the statistical structure of prey time series
BMC Ecology (2009)
-
Relationships between the emergence and oviposition of ectoparasitoid Spathius agrili Yang and its host emerald ash borer, Agrilus planipennis Fairmaire
Frontiers of Forestry in China (2007)
-
Single-species models for many-species food webs
Nature (2002)
-
Bagging the lag
Nature (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.