Abstract
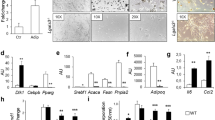
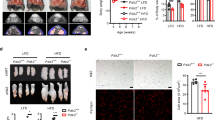
The earliest defect in developing type 2 diabetes is insulin resistance1,2, characterized by decreased glucose transport and metabolism in muscle and adipocytes3,4. The glucose transporter GLUT4 mediates insulin-stimulated glucose uptake in adipocytes and muscle by rapidly moving from intracellular storage sites to the plasma membrane4. In insulin-resistant states such as obesity and type 2 diabetes, GLUT4 expression is decreased in adipose tissue but preserved in muscle3,4. Because skeletal muscle is the main site of insulin-stimulated glucose uptake, the role of adipose tissue GLUT4 downregulation in the pathogenesis of insulin resistance and diabetes is unclear. To determine the role of adipose GLUT4 in glucose homeostasis, we used Cre/loxP DNA recombination to generate mice with adipose-selective reduction of GLUT4 (G4A-/-). Here we show that these mice have normal growth and adipose mass despite markedly impaired insulin-stimulated glucose uptake in adipocytes. Although GLUT4 expression is preserved in muscle, these mice develop insulin resistance in muscle and liver, manifested by decreased biological responses and impaired activation of phosphoinositide-3-OH kinase. G4A-/- mice develop glucose intolerance and hyperinsulinaemia. Thus, downregulation of GLUT4 and glucose transport selectively in adipose tissue can cause insulin resistance and thereby increase the risk of developing diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cline, G. W. et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–246 (1999).
Roden, M. & Shulman, G. I. Applications of NMR spectroscopy to study muscle glycogen metabolism in man. Annu. Rev. Med. 50, 277–290 (1999).
Defronzo, R. A. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 5, 177–269 (1997).
Shepherd, P. R. & Kahn, B. B. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341, 248–257 (1999).
Abel, E. D. et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J. Clin. Invest. 104, 1703–1714 (1999).
Zisman, A. et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nature Med. 6, 924–928 (2000).
Ross, S. R. et al. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc. Natl Acad. Sci. USA 87, 9590–9504 (1990).
Zambrowicz, B. P. et al. Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl Acad. Sci. USA 94, 3789–3794 (1997).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999).
Katz, E. B., Stenbit, A. E., Hatton, K., Depinho, R. & Charron, M. J. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377, 151–155 (1995).
Donoghue, M. J., Alvarez, J. D., Merlie, J. P. & Sanes, J. R. Fiber type- and position-dependent expression of a myosin light chain- CAT transgene detected with a novel histochemical stain for CAT. J. Cell Biol. 115, 423–434 (1991).
West, D. B., Boozer, C. N., Moody, D. L. & Atkinson, R. L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. 262, R1025–R1032 (1992).
Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46, 3–10 (1997).
Muoio, D. M. et al. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46, 1360–1363 (1997).
Burcelin, R. et al. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes 48, 1264–1269 (1999).
Hotamisligil, G. S. & Spiegelman, B. M. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes 43, 1271–1278 (1994).
Griffin, M. E. et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48, 1270–1274 (1999).
Dresner, A. et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103, 253–9 (1999).
Mueller, W. M. et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 139, 551–558 (1998).
Sivitz, W. I., Walsh, S. A., Morgan, D. A., Thomas, M. J. & Haynes, W. G. Effects of leptin on insulin sensitivity in normal rats. Endocrinology 138, 3395–3401 (1997).
Tozzo, E., Gnudi, L. & Kahn, B. B. Amelioration of insulin resistance in streptozotocin diabetic mice by transgenic overexpression of GLUT4 driven by an adipose-specific promoter. Endocrinology 138, 1604–1611 (1997).
Cushman, S. W. & Salans, L. B. Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J. Lipid Res. 19, 269–273 (1978).
Kim, J. K., Gavrilova, O., Chen, Y., Reitman, M. L. & Shulman, G. I. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 275, 8456–8460 (2000).
Kim, Y. B. et al. Glucosamine infusion in rats rapidly impairs insulin stimulation of phosphoinositide 3-kinase but does not alter activation of Akt/protein kinase B in skeletal muscle. Diabetes 48, 310–320 (1999).
Storlien, L. H. et al. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40, 280–289 (1991).
Acknowledgements
This work was supported by NIH Grants (to E.D.A., G.I.S. and B.B.K.) and an American Diabetes Association award to B.B.K. E.D.A. was the recipient of awards from the Robert Wood Johnson Foundation and the Eleanor and Miles Shore Scholars in Medicine Fellowship (Harvard Medical School). O.P. was supported by grants from the ALFEDIAM Society and Nestlé, France; and O.B. by the Human Frontier Sciences Program. We thank H. Chen for performing the TaqMan assays; P. She and M.A. Magnusen for breeding aP2–Cre mice with Rosa26–lacZ floxed mice; G. Hotamisligil for helpful advice; and C. Oberste-Berghaus and M. Pham for expert assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abel, E., Peroni, O., Kim, J. et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 (2001). https://doi.org/10.1038/35055575
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35055575
This article is cited by
-
Flavonoid compound from Agrimonia pilosa Ledeb improves adipose insulin resistance by alleviating oxidative stress and inflammation
BMC Complementary Medicine and Therapies (2023)
-
Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment
Journal of Ovarian Research (2023)
-
Lipid remodeling of adipose tissue in metabolic health and disease
Experimental & Molecular Medicine (2023)
-
Phosphoproteomics reveals rewiring of the insulin signaling network and multi-nodal defects in insulin resistance
Nature Communications (2023)
-
Unraveling the rationale and conducting a comprehensive assessment of KD025 (Belumosudil) as a candidate drug for inhibiting adipogenic differentiation—a systematic review
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.