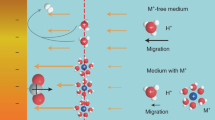
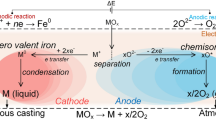
Abstract
Many reactive metals are difficult to prepare in pure form without complicated and expensive procedures1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18. Although titanium has many desirable properties (it is light, strong and corrosion-resistant1), its use has been restricted because of its high processing cost. In the current pyrometallurgical process—the Kroll process4,5—the titanium minerals rutile and ilmenite are carbo-chlorinated to remove oxygen, iron and other impurities, producing a TiCl4 vapour. This is then reduced to titanium metal by magnesium metal; the by-product MgCl2 is removed by vacuum distillation. The prediction that this process would be replaced by an electrochemical route6,7,8,9,10 has not been fulfilled; attempts involving the electro-deposition of titanium from ionic solutions have been hampered by difficulties in eliminating the redox cycling of multivalent titanium ions and in handling very reactive dendritic products6,7,8,9,10. Here we report an electrochemical method for the direct reduction of solid TiO2, in which the oxygen is ionized, dissolved in a molten salt and discharged at the anode, leaving pure titanium at the cathode. The simplicity and rapidity of this process compared to conventional routes should result in reduced production costs and the approach should be applicable to a wide range of metal oxides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Habashi, F. (ed.) Handbook of Extractive Metallurgy 1129–1180 (Wiley-VCH, Weinham, 1997).
Sadoway, D. R. in Refractory Metals Extraction, Processing and Applications: Proc. TMS Annual Meeting, New Orleans 1991 (eds Liddel, K. C., Sadoway, D. R. & Bautista, R. G.) (TMS—The Minerals, Metals & Materials Society, Warrendale, 1991).
Massalski, T. B., Okamoto, H., Subramanian, P. R. & Kacprzak, L. (eds) Binary Alloy Phase Diagrams 2nd edn, Vol. 3, 2924–2927 (ASM International, Materials Park, 1990).
Kroll, W. J. The production of ductile titanium. Trans. Am. Electrochem. Soc. 78, 35–47 ( 1940).
Ikeshima, T., in Titanium Science and Technology, Proc. 5th Int. Conf. Titanium, München 1984 (eds Lutjering, G., Zwicker, U. & Bunk, W.) 3– 14 (DGM-Deutsche Gesellschaft für Materialkunde e.V., Oberursal, 1985).
Cobel, G., Fisher, J. & Synder, L. E. in Titanium ’80, Science and Technology, Proc. 4th Int. Conf. Titanium, Kyoto 1980 (eds Kimura, H. & Izumi, O.) 1969–1976 (The Metallurgical Society of AIME, Warrendale, 1980).
Opie, W. R. & Moles, O. W. A basket cathode electrolytic cell for production of titanium. Trans. Met. Soc. AIME 218 , 646–649 (1960).
Ginatta, M. V. Method of producing metals by cathodic dissolution of their compounds. US Patent 4,400,247 (23 Aug. 1983).
Froes, F. H. Titanium and other light metals: let's do something about cost. JOM 50, 15 (1998).
Hartman, A. D., Gerdemann, S. J. & Hansen, J. S. Producing lower-cost titanium for automotive applications. JOM 50, 16–19 (1998).
Suzuki, K. The high-quality precision casting of titanium alloys. JOM 50, 20–23 (1998).
Okabe, T., Ohkubo, C., Watanabe, I., Okuno, O. & Takada, Y. The present status of dental titanium casting. JOM 50, 24–29 ( 1998).
Froes, F. H. The production of low-cost titanium powders. JOM 50 , 41–43 (1998).
Tapphorn, R. M. & Gabel, H. The solid-state spray forming of low-oxide titanium components. JOM 50, 45–46, 76 (1998).
Elliott, G. R. B. The continuous production of titanium powder using circulating molten salt. JOM 50, 48–49 (1998).
Sohn, H. Y. Ti and TiAl powders by the flash reduction of chloride vapors. JOM 50, 50–51 ( 1998).
Segall, A. E., Papyrin, A. N., Conway, J. C. Jr & Shapiro, D. A cold-gas spray coating process for enhancing titanium. JOM 50, 52–54 (1998).
Larson, H. R. & Eagar, T. W. The plasma-enhanced recovery of titanium by the electrolysis of titanate slags. JOM 50, 56–57 (1998).
Okabe, T. H., Oishi, T. & Ono, K. Deoxidation of titanium aluminide by Ca-Al alloy under controlled aluminum activity. Metall. Trans. B 23, 583– 590 (1992).
Okabe, T. H., Deura, T., Oishi, T., Ono, K. & Sadoway, D. R. Thermodynamic properties of oxygen in yttrium-oxygen solid solutions. J. Alloys Compounds 237, 841–847 (1996).
Boghosian, S., Godo, Aa., Mediaas, H., Ravlo, W. & Ostvold, T. Oxide complexes in alkali alkaline-earth chloride melts. Acta Chem. Scand. 45, 145– 157 (1991).
Gruber, H. & Krautz, E. Magnetoresistance and conductivity in the binary-system titanium oxygen. 2. Semiconductive titanium-oxides. Phys. Status Solidi A 69, 287–295 (1982).
McQuillan, A. D. & McQuillan, M. K. Titanium 402–426 (Butterworths Scientific, London, 1956).
McKee, D. W. Gasification of graphite in carbon-dioxide and water-vapour—the catalytic effects of alkali-metal salts. Carbon 20, 59–66 (1982).
Acknowledgements
This research was sponsored by the EPSRC. T.W.F. first suggested the electrochemical deoxidation of titanium metal. G.Z.C. was the first to observe that it was possible to reduce thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Fray, D. & Farthing, T. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407, 361–364 (2000). https://doi.org/10.1038/35030069
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35030069
This article is cited by
-
Development of deoxidation process for off-grade titanium sponge using magnesium metal with wire mesh strainer type of crucible
Scientific Reports (2024)
-
An Electrolysis-Distillation Approach for Producing Potassium Metal
Metallurgical and Materials Transactions B (2024)
-
A novel method for extracting metals from asteroids using non-aqueous deep eutectic solvents
Scientific Reports (2023)
-
An iron-base oxygen-evolution electrode for high-temperature electrolyzers
Nature Communications (2023)
-
Thermodynamic and kinetic considerations of nitrogen carriers for chemical looping ammonia synthesis
Discover Chemical Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.