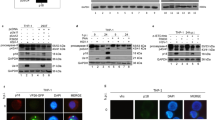
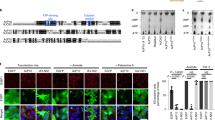
Abstract
Successful viral infection requires viruses to redirect host biochemistry to replicate the viral genome, and produce and assemble progeny virions. Cellular heat-shock responses, which are characterized as elevation and relocalization of heat-shock proteins, occur during replication of many viruses1,2,3,4,5,6,7. Such responses might be host reactions to the synthesis of foreign protein, or might be irrelevant consequences of the viral need to activate transcription. Alternatively, as heat-shock proteins can facilitate protein folding8,9, activating a heat-shock response might be a specific virus function ensuring proper synthesis of viral proteins and virions. It is not possible to determine whether heat-shock response is essential for virus replication, because the implicated viral genes (such as Ad5 E1A, ref. 10) also control other essential replication steps. Here we report that expression of Gam1, a protein encoded by the avian virus CELO (ref. 11), elevates and relocalizes hsp70 and hsp40. Gam1-negative CELO is replication-defective; however, Gam1 function can be partially replaced by either heat shock or forced hsp40 expression. Thus, an essential function of Gam1 during virus replication is to activate host heat-shock responses with hsp40 as a primary target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nevins, J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell 29, 913–919 (1982).
Kao, H. T. & Nevins, J. R. Transcriptional activation and subsequent control of the human heat shock gene during adenovirus infection. Mol. Cell. Biol. 3, 2058– 2065 (1983).
Phillips, B., Abravaya, K. & Morimoto, R. I. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. J. Virol. 65, 5680–5692 (1991).
Santomenna, L. D. & Colberg-Poley, A. M. Induction of cellular hsp70 expression by human cytomegalovirus. J. Virol. 64, 2033–2040 ( 1990).
Ohgitani, E., Kobayashi, K., Takeshita, K. & Imanishi, J. Biphasic translocation of a 70 kDa heat shock protein in human cytomegalovirus-infected cells. J. Gen. Virol. 80, 63– 68 (1999).
Kobayashi, K. et al. Herpes simplex virus-induced expression of 70 kDa heat shock protein (HSP70) requires early protein synthesis but not viral DNA replication. Microbiol. Immunol. 38, 321– 325 (1994).
Collins, P. L. & Hightower, L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J. Virol. 44, 703–707 ( 1982).
Mayer, M. P. & Bukau, B. HSP70 chaperone systems: diversity of cellular functions and mechanism of action. Biol. Chem. 379, 261–268 (1998).
Kelley, W. L. Molecular chaperones: How J domains turn on Hsp70s. Curr. Biol. 9, R305–308 ( 1999).
Flint, J. & Shenk, T. Viral transactivating proteins. Annu. Rev. Genet. 31, 177–212 (1997).
Chiocca, S., Baker, A. & Cotten, M. Identification of a novel anti-apoptotic protein, GAM-1, encoded by the CELO adenovirus. J. Virol. 71, 3168–3177 (1997).
Gabai, V. L. et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J. Biol. Chem. 272, 18033–18037 (1997).
Mosser, D. D. et al. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17, 5317–5327 (1997).
Welch, W. J. & Feramisco, J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J. Biol. Chem. 259, 4501–4513 (1984)
Hattori, H. et al. Intracellular localization and partial amino acid sequence of a stress-inducible 40-kDa protein in HeLa cells. Cell. Struct. Funct. 17, 77–86 ( 1992).
Polissi, A., Goffin, L. & Georgopoulos, C. The Escherichia coli heat shock response and bacteriophage lambda development. FEMS Microbiol. Rev. 17, 159–169 (1995).
Liu, J. S. et al. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 273, 30704 –30712 (1998).
Campbell, K. S. et al. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 11, 1098–1110 (1997).
Kelley, W. L. & Georgopoulos, C. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc. Natl Acad. Sci. USA 94, 3679– 3684 (1997).
Stubdal, H. et al. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol. Cell. Biol. 17, 4979–4990 ( 1997).
Srinivasan, A. et al. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 17, 4761–4773 ( 1997).
Chiocca, S. et al. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J. Virol. 70, 2939 –2949 (1996).
Chartier, C. et al. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70, 4805–4810 ( 1996).
Michou, A. -I., Lehrmann, H., Saltik, M. & Cotten, M. Mutational analysis of the avian adenovirus CELO, which provides a basis for gene delivery vectors. J. Virol. 73, 1399–1410 (1999).
Ohtsuka, K. Cloning of a cDNA for heat-shock protein hsp40, a human homologue of bacterial DnaJ. Biochem. Biophys. Res. Commun. 197, 235–240 (1993).
Hunt, C. & Morimoto, R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl Acad. Sci. USA 82, 6455– 6459 (1985).
Stratford-Perricaudet, L. D., Makeh, I., Perricaudet, M. & Briand, P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J. Clin. Invest. 90, 626–630 (1992).
Evan, G. I., Lewis, G. K., Ramsay, G. & Bishop, J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610–3616 (1985).
Kawaguchi T., Nomura, K., Hirayama, Y. & Kitagawa, T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 47, 4460–4464 (1987).
Graham F. L., Smiley, J., Russell, W. C. & Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59– 74 (1977).
Acknowledgements
We thank M. Raff for suggesting the heat-shock complementation and G. Christofori for his comments on the manuscript. We thank K. Ohtsuka for the hsp40 cDNA and S. Fox and R. Morimoto for the hsp70 gene.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glotzer, J., Saltik, M., Chiocca, S. et al. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature 407, 207–211 (2000). https://doi.org/10.1038/35025102
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35025102
This article is cited by
-
Heat shock protein 70 could enhance porcine epidemic diarrhoea virus replication by interacting with membrane proteins
Veterinary Research (2021)
-
Diversity in heat shock protein families: functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle
Cell Stress and Chaperones (2021)
-
Porcine DNAJB6 promotes PCV2 replication via enhancing the formation of autophagy in host cells
Veterinary Research (2020)
-
High basal heat-shock protein expression in bats confers resistance to cellular heat/oxidative stress
Cell Stress and Chaperones (2019)
-
A furoviral replicase recruits host HSP70 to membranes for viral RNA replication
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.