Abstract
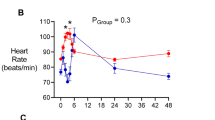
Vertebrates achieve internal homeostasis during infection or injury by balancing the activities of proinflammatory and anti-inflammatory pathways. Endotoxin (lipopolysaccharide), produced by all gram-negative bacteria, activates macrophages to release cytokines that are potentially lethal1,2,3,4. The central nervous system regulates systemic inflammatory responses to endotoxin through humoral mechanisms5,6,7,8. Activation of afferent vagus nerve fibres by endotoxin or cytokines stimulates hypothalamic–pituitary–adrenal anti-inflammatory responses9,10,11. However, comparatively little is known about the role of efferent vagus nerve signalling in modulating inflammation. Here, we describe a previously unrecognized, parasympathetic anti-inflammatory pathway by which the brain modulates systemic inflammatory responses to endotoxin. Acetylcholine, the principle vagal neurotransmitter, significantly attenuated the release of cytokines (tumour necrosis factor (TNF), interleukin (IL)-1β, IL-6 and IL-18), but not the anti-inflammatory cytokine IL-10, in lipopolysaccharide-stimulated human macrophage cultures. Direct electrical stimulation of the peripheral vagus nerve in vivo during lethal endotoxaemia in rats inhibited TNF synthesis in liver, attenuated peak serum TNF amounts, and prevented the development of shock.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tracey, K. J. et al. Shock and tissue injury induced by recombinant human cachectin. Science 234, 470–474 (1986).
Dinarello, C. A. The interleukin-1 family: 10 years of discovery. FASEB J. 8, 1314–1325 (1994).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 ( 1999).
Nathan, C. F. Secretory products of macrophages. J. Clin. Invest. 79, 319–326 (1987).
Besedovsky, H., Rey, D. A., Sorkin, E. & Dinarello, C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654 ( 1986).
Woiciechowsky, C. et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nature Med. 4, 808–813 (1998).
Hu, X. X., Goldmuntz, E. A. & Brosnan, C. F. The effect of norepinephrine on endotoxin-mediated macrophage activation. J. Neuroimmunol. 31, 35–42 (1991).
Lipton, J. M. & Catania, A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol. Today 18, 140–145 (1997).
Watkins, L. R. & Maier, S. F. Implications of immune-to-brain communication for sickness and pain. Proc. Natl Acad. Sci. USA 96, 7710–7713 ( 1999).
Sternberg, E. M. Neural-immune interactions in health and disease. J. Clin. Invest. 100, 2641–2647 ( 1997).
Scheinman, R. I., Cogswell, P. C., Lofquist, A. K. & Baldwin, A. S. Jr Role of transcriptional activation of IkappaB alpha in mediation of immunosuppression by glucocorticoids. Science 270, 283– 286 (1995).
Gaykema, R. P., Dijkstra, I. & Tilders, F. J. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology 136, 4717– 4720 (1995).
Fleshner, M. et al. Thermogenic and corticosterone responses to intravenous cytokines (IL-1beta and TNF-alpha) are attenuated by subdiaphragmatic vagotomy. J. Neuroimmunol. 86, 134–141 (1998).
Watkins, L. R. et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 183, 27–31 (1995).
Romanovsky, A. A., Simons, C. T., Szekely, M. & Kulchitsky, V. A. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. 273, R407– R413 (1997).
Antonica, A., Magni, F., Mearini, L. & Paolocci, N. Vagal control of lymphocyte release from rat thymus. J. Auton. Nerv. Syst. 48, 187–197 (1994).
Guslandi, M. Nicotine treatment for ulcerative colitis. Br. J. Clin. Pharmacol. 48, 481–484 ( 1999).
Sandborn, W. J. et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 126, 364–371 (1997).
Sher, M. E. et al. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 5, 73–78 (1999).
Sato, E., Koyama S., Okubo, Y., Kubo, K. & Sekigushi, M. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am. J. Physiol. 274, L970–L979 (1998).
Whaley, K., Lappin, D. & Barkas, T. C2 synthesis by human monocytes is modulated by a nicotinic cholinergic receptor. Nature 293, 580– 583 (1981).
Sato, K. Z. et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci. Lett. 266, 17–20 ( 1999).
Wathey, J. C., Nass, M. M. & Lester, H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys. J. 27, 145– 164 (1979).
Kumins, N. H., Hunt, J., Gamelli, R. L. & Filkins, J. P. Partial hepatoectomy reduces the endotoxin-induced peak circulating level of tumor necrosis factor in rats. Shock 5, 385–388 (1996).
Gregory, S. H. & Wing, E. J. Neutrophil-Kupffer-cell interaction in host defenses to systemic infections. Immunol. Today 19, 507–510 ( 1998).
Tracey, K. J. et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662 –664 (1987).
Madretsma, G. S. et al. Nicotine inhibits the in vitro production of interleukin 2 and tumour necrosis factor-alpha by human mononuclear cells. Immunopharmacology 35, 47–51 ( 1996).
van Dijk, A. P. et al. Transdermal nicotine inhibits interleukin 2 synthesis by mononuclear cells derived from healthy volunteers. Eur. J. Clin. Invest. 28, 664–671 (1998).
Acknowledgements
We thank D. Nardi for help in ELISA assays and D. Prieto for administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borovikova, L., Ivanova, S., Zhang, M. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). https://doi.org/10.1038/35013070
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35013070
This article is cited by
-
The gateway reflex regulates tissue-specific autoimmune diseases
Inflammation and Regeneration (2024)
-
A novel neuroimmune modulation system for the treatment of rheumatoid arthritis
Bioelectronic Medicine (2024)
-
Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation
Journal of Neuroinflammation (2024)
-
The immunomodulatory effect of oral NaHCO3 is mediated by the splenic nerve: multivariate impact revealed by artificial neural networks
Journal of Neuroinflammation (2024)
-
Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.