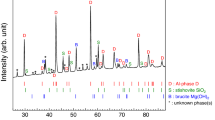
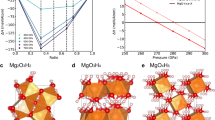
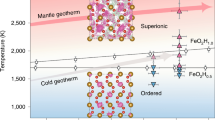
Abstract
WATER locked in mineral phases within the Earth's mantle may be as significant for the development of life as water now found at the planet's surface. The Earth's original hydrosphere probably would not have survived a collision with a Mars-sized object, such as may have formed the Moon1. The water now on the surface could have been replenished by further impacts of smaller planetesimals; however, at least a portion could have been stored in minerals deep in the mantle being released gradually through volcanic eruptions. This mechanism requires a stable high-pressure phase able to store water under mantle conditions. Of the several hydrous phases studied in the past, the material known as phase B has the highest density and is the only known hydrous form stable at pressures corresponding to depths of 400–500 km (refs 2, 3). Although phase B has been known for over twenty years, its crystal structure and crystal chemistry have remained an unsolved problem. Here we describe the crystal structures of phase B (Mg12Si4O19(OH) 2) and a chemically and structurally similar anhydrous form, AnhB (Mg14Si5O24). These structures contain silicon in both fourfold and sixfold coordination; the silicon octahedra share all twelve edges with magnesium octahedra in a unique thirteen-cation cluster. Description of the structures of phases AnhB and B requires 18 and 40 atoms, respectively, demonstrating that high-pressure phases can have very complicated crystal structures. The multitude of octahedral sites in these phases could lead to rather complicated fractionation behaviour during solid–solid or solid–liquid transitions in the presence of other octahedrally coordinated ions such as Al, Fe, Ti and Mn.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Newson, H. E. & Taylor, S. R. Nature 338, 29–34 (1989).
Ringwood, A. E. & Major, A. Earth planet. Sci. Lett. 22, 130–133 (1967).
Akaogi, M. & Akimoto, S. J. geophys. Res. 84, 6944–6948 (1980).
Akaogi, M. & Akimoto, S. Phys. Chem. Miner. 13, 161–164 (1986).
Akimoto, S. & Akaogi, M. in Materials Science of the Earth's Interior (ed. Sunagawa, I.) 477–480 (Terra Scientific, Tokyo, 1984).
Kato, T. & Kumazawa, M. Geophys. Res. Lett. 13, 181–184 (1986).
Kato, T. & Kumazawa, M. Geophys. Res. Lett. 12, 534–535 (1985).
Herzberg, C. T. & Gasparik, T. Eos 70, 484 (1989).
Gilmore, G. J. J. appl. Crystallogr. 17, 42–46 (1984).
von Dreele, R. B., Bless, P. W., Kostiner, E. & Hughes, R. E. J. Solid St. Chem. 2, 612–618 (1970).
Brown, I. D. in Structure and Bonding in Crystals II (eds O'Keeffe, M. & Navrotsky, A.) 1–30 (Academic, New York, 1981).
Liu, L. Phys. Earth planet. Inter. 42, 255–262 (1986).
Papike, J. J. & Cameron, M. Rev. Geophys. Space Phys. 14, 37–80 (1976).
Horiuchi, H., Morimoto, N., Yamamoto, K. & Akimoto, S. Am. Miner. 64, 593–598 (1979).
Barbier, J. Acta crystallogr. B43, 422–429 (1987).
Barbier, J. J. Solid St. Chem. 68, 52–60 (1987).
Veblen, D. R. & Buseck, P. R. Am. Miner. 64, 687–700 (1979).
McGetchen, T. R., Silver, L. T. & Chodos, A. A. J. geophys. Res. 75, 255–259 (1970).
Matsui, T., & Abe, Y. Nature 322, 526–528 (1986).
Fisher, R. X. J. appl. Crystallogr. 18, 258–262 (1984).
Morimoto, N., Tokonomi, M., Watanabe, M. & Koto, K. Am. Miner. 59, 475–485 (1974).
Lin, L. Geophys. Res. Lett. 9, 124–126 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Finger, L., Ko, J., Hazen, R. et al. Crystal chemistry of phase B and an anhydrous analogue: implications for water storage in the upper mantle. Nature 341, 140–142 (1989). https://doi.org/10.1038/341140a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/341140a0
This article is cited by
-
Behaviour of Fe4O5–Mg2Fe2O5 solid solutions and their relation to coexisting Mg–Fe silicates and oxide phases
Contributions to Mineralogy and Petrology (2018)
-
The stability of anhydrous phase B, Mg14Si5O24, at mantle transition zone conditions
Physics and Chemistry of Minerals (2018)
-
Chromium solubility in anhydrous Phase B
Physics and Chemistry of Minerals (2016)
-
Hydrogen sites in the dense hydrous magnesian silicate phase E: a pulsed neutron powder diffraction study
Physics and Chemistry of Minerals (2016)
-
Buoyancy-driven propagation of an isolated fluid-filled crack in rock: implication for fluid transport in metamorphism
Contributions to Mineralogy and Petrology (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.