Abstract
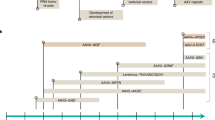
Duchenne muscular dystrophy (DMD) is a severe muscle wasting disorder affecting 1/3500 male births. There is currently no effective treatment, but gene therapy approaches are offering viable avenues for treatment development. The last 10 years have seen the development of a number of strategies and tools for muscle gene therapy. However, the major hurdle has been the inability to deliver vectors at high enough efficiency via a systemic route. The last 2–3 years (reviewed here) have seen unrivalled progress in efficient systemic delivery of viral and non-viral gene transfer agents and antisense oligonucleotides. This progress, coupled with the successful completion of the first gene therapy clinical trial for DMD, has led to three more clinical trials planned for the immediate future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Romero NB, Braun S, Benveniste O, Leturcq F, Hogrel JY, Morris GE et al. Phase I study of dystrophin plasmid-based gene therapy in Duchenne/Becker muscular dystrophy. Hum Gene Ther 2004; 15: 1065–1076.
Lu QL, Bou-Gharios G, Partridge TA . Non-viral gene delivery in skeletal muscle: a protein factory. Gene Therapy 2003; 10: 131–142.
Garg S, Oran AE, Hon H, Jacob J . The hybrid cytomegalovirus enhancer/chicken beta-actin promoter along with woodchuck hepatitis virus posttranscriptional regulatory element enhances the protective efficacy of DNA vaccines. J Immunol 2004; 173: 550–558.
Hlavaty J, Schittmayer M, Stracke A, Jandl G, Knapp E, Felber BK et al. Effect of posttranscriptional regulatory elements on transgene expression and virus production in the context of retrovirus vectors. Virology 2005; 341: 1–11.
Kingsman SM, Mitrophanous K, Olsen JC . Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE). Gene Therapy 2005; 12: 3–4.
Nash KL, Jamil B, Maguire AJ, Alexander GJ, Lever AM . Hepatocyte-specific gene expression from integrated lentiviral vectors. J Gene Med 2004; 6: 974–983.
Azzouz M, Ralph S, Wong LF, Day D, Askham Z, Barber RD et al. Neuroprotection in a rat Parkinson model by GDNF gene therapy using EIAV vector. Neuroreport 2004; 15: 985–990.
Garmory HS, Brown KA, Titball RW . DNA vaccines: improving expression of antigens. Genet Vaccines Ther 2003; 1: 2.
Richard P, Bossard F, Desigaux L, Lanctin C, Bello-Roufai M, Pitard B . Amphiphilic block copolymers promote gene delivery in vivo to pathological skeletal muscles. Hum Gene Ther 2005; 16: 1318–1324.
Lu QL, Liang HD, Partridge T, Blomley MJ . Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Therapy 2003; 10: 396–405.
Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV . Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation 2003; 108: 1022–1026.
Bloquel C, Fabre E, Bureau MF, Scherman D . Plasmid DNA electrotransfer for intracellular and secreted proteins expression: new methodological developments and applications. J Gene Med 2004; 6 (Suppl 1): S11–S23.
Andre F, Mir LM . DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Therapy 2004; 11 (Suppl 1): S33–S42.
Ferrer A, Foster H, Wells KE, Dickson G, Wells DJ . Long-term expression of full-length human dystrophin in transgenic mdx mice expressing internally deleted human dystrophins. Gene Therapy 2004; 11: 884–893.
Leroy-Willig A, Bureau MF, Scherman D, Carlier PG . In vivo NMR imaging evaluation of efficiency and toxicity of gene electrotransfer in rat muscle. Gene Therapy 2005; 12: 1434–1443.
Bertoni C, Jarrahian S, Wheeler TM, Li Y, Olivares EC, Calos MP et al. Enhancement of plasmid-mediated gene therapy for muscular dystrophy by directed plasmid integration. Proc Natl Acad Sci USA 2006; 103: 419–424.
Danialou G, Comtois AS, Matecki S, Nalbantoglu J, Karpati G, Gilbert R et al. Optimization of regional intra-arterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol Ther 2005; 11: 257–266.
Liang KW, Nishikawa M, Liu F, Sun B, Ye Q, Huang L . Restoration of dystrophin expression in mdx mice by intravascular injection of naked DNA containing full-length dystrophin cDNA. Gene Therapy 2004; 11: 901–908.
Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H et al. Intra-arterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther 2004; 15: 770–782.
Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther 2004; 10: 386–398.
Athanasopoulos T, Graham IR, Foster H, Dickson G . Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD). Gene Therapy 2004; 11 (Suppl 1): S109–S121.
Blankinship MJ, Gregorevic P, Chamberlain JS . Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated virus vectors. Mol Ther 2006; 13: 241–249.
Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D . Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 2005; 11: 245–256.
Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther 2004; 10: 821–828.
Gao G, Vandenberghe LH, Wilson JM . New recombinant serotypes of AAV vectors. Curr Gene Ther 2005; 5: 285–297.
Louboutin JP, Wang L, Wilson JM . Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med 2005; 7: 442–451.
Gonin P, Arandel L, Van Wittenberghe L, Marais T, Perez N, Danos O . Femoral intra-arterial injection: a tool to deliver and assess recombinant AAV constructs in rodents whole hind limb. J Gene Med 2005; 7: 782–791.
Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 2004; 306: 1796–1799.
Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation 2005; 112: 1780–1788.
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004; 10: 828–834.
Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA . Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol 2005; 79: 214–224.
Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005; 23: 321–328.
Maheshri N, Koerber JT, Kaspar BK, Schaffer DV . Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol 2006; 24: 198–204.
Muzyczka N, Warrington Jr KH . Custom adeno-associated virus capsids: the next generation of recombinant vectors with novel tropism. Hum Gene Ther 2005; 16: 408–416.
Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 2006; 107: 2653–2661.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004; 78: 6381–6388.
Schmidt M, Katano H, Bossis I, Chiorini JA . Cloning and characterization of a bovine adeno-associated virus. J Virol 2004; 78: 6509–6516.
Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L et al. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther 2006; 13: 77–87.
Miller DG, Trobridge GD, Petek LM, Jacobs MA, Kaul R, Russell DW . Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol 2005; 79: 11434–11442.
Pachori AS, Melo LG, Zhang L, Loda M, Pratt RE, Dzau VJ . Potential for germ line transmission after intramyocardial gene delivery by adeno-associated virus. Biochem Biophys Res Commun 2004; 313: 528–533.
Ghosh A, Yue Y, Duan D . Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J Gene Med 2006; 8: 298–305.
Xu Z, Yue Y, Lai Y, Ye C, Qiu J, Pintel DJ et al. Trans-splicing adeno-associated viral vector-mediated gene therapy is limited by the accumulation of spliced mRNA but not by dual vector coinfection efficiency. Hum Gene Ther 2004; 15: 896–905.
Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol 2005; 23: 1435–1439.
Aartsma-Rus A, De Winter CL, Janson AA, Kaman WE, Van Ommen GJ, Den Dunnen JT et al. Functional analysis of 114 exon-internal AONs for targeted DMD exon skipping: indication for steric hindrance of SR protein binding sites. Oligonucleotides 2005; 15: 284–297.
Graham IR, Hill VJ, Manoharan M, Inamati GB, Dickson G . Towards a therapeutic inhibition of dystrophin exon 23 splicing in mdx mouse muscle induced by antisense oligoribonucleotides (splicomers): target sequence optimisation using oligonucleotide arrays. J Gene Med 2004; 6: 1149–1158.
Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med 2003; 9: 1009–1014.
Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, den Dunnen JT, Baas F et al. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Hum Mol Genet 2003; 12: 907–914.
Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, van Ommen GJ, den Dunnen JT et al. Antisense-induced multiexon skipping for Duchenne muscular dystrophy makes more sense. Am J Hum Genet 2004; 74: 83–92.
Torelli S, Brown SC, Jimenez-Mallebrera C, Feng L, Muntoni F, Sewry CA . Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol Appl Neurobiol 2004; 30: 540–545.
Bremmer-Bout M, Aartsma-Rus A, de Meijer EJ, Kaman WE, Janson AA, Vossen RH et al. Targeted exon skipping in transgenic hDMD mice: a model for direct preclinical screening of human-specific antisense oligonucleotides. Mol Ther 2004; 10: 232–240.
Aartsma-Rus A, Kaman WE, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ et al. Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells. Gene Therapy 2004; 11: 1391–1398.
Surono A, Van Khanh T, Takeshima Y, Wada H, Yagi M, Takagi M et al. Chimeric RNA/ethylene-bridged nucleic acids promote dystrophin expression in myocytes of Duchenne muscular dystrophy by inducing skipping of the nonsense mutation-encoding exon. Hum Gene Ther 2004; 15: 749–757.
Yagi M, Takeshima Y, Surono A, Takagi M, Koizumi M, Matsuo M . Chimeric RNA and 2′-O, 4′-C-ethylene-bridged nucleic acids have stronger activity than phosphorothioate oligodeoxynucleotides in induction of exon 19 skipping in dystrophin mRNA. Oligonucleotides 2004; 14: 33–40.
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med 2006; 12: 175–177.
Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Wilton SD . Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med 2006; 8: 207–216.
Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA 2005; 102: 198–203.
Brun C, Suter D, Pauli C, Dunant P, Lochmuller H, Burgunder JM et al. U7 snRNAs induce correction of mutated dystrophin pre-mRNA by exon skipping. Cell Mol Life Sci 2003; 60: 557–566.
Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C et al. Body-wide gene therapy of Duchenne muscular dystrophy in the mdx mouse model. Proc Natl Acad Sci USA 2006; 103: 3758–3763.
Muntoni F, Bushby K, van Ommen G . 128th ENMC International Workshop on ‘Preclinical Optimization and Phase I/II Clinical Trials Using Antisense Oligonucleotides in Duchenne Muscular Dystrophy’ 22–24 October 2004, Naarden, The Netherlands. Neuromuscul Disord 2005; 15: 450–457.
Goldspink G . Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 2005; 20: 232–238.
Goldspink G . Impairment of IGF-I gene splicing and MGF expression associated with muscle wasting. Int J Biochem Cell Biol 2006; 38: 481–489.
Shavlakadze T, Winn N, Rosenthal N, Grounds MD . Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm IGF Res 2005; 15: 4–18.
Tidball JG . Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol 2005; 98: 1900–1908.
Glass DJ . Molecular mechanisms modulating muscle mass. Trends Mol Med 2003; 9: 344–350.
Sartorelli V, Fulco M . Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE 2004; 2004: re11.
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL . IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 2004; 287: E591–E601.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004; 117: 399–412.
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004; 14: 395–403.
Haddad F, Baldwin KM, Tesch PA . Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 2005; 98: 46–52.
Hill M, Wernig A, Goldspink G . Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 2003; 203: 89–99.
Yang SY, Goldspink G . Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 2002; 522: 156–160.
Sacco A, Doyonnas R, LaBarge MA, Hammer MM, Kraft P, Blau HM . IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J Cell Biol 2005; 171: 483–492.
Abmayr S, Gregorevic P, Allen JM, Chamberlain JS . Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol Ther 2005; 12: 441–450.
Chen ZY, He CY, Ehrhardt A, Kay MA . Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther 2003; 8: 495–500.
Chen ZY, He CY, Kay MA . Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther 2005; 16: 126–131.
Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 2003; 300: 965–971.
Wagner KR, Liu X, Chang X, Allen RE . Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA 2005; 102: 2519–2524.
McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L et al. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 2005; 118: 3531–3541.
Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS . Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 2005; 19: 543–549.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foster, K., Foster, H. & Dickson, J. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther 13, 1677–1685 (2006). https://doi.org/10.1038/sj.gt.3302877
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302877
Keywords
This article is cited by
-
Episomal minicircles persist in periods of transcriptional inactivity and can be transmitted through somatic cell nuclear transfer into bovine embryos
Molecular Biology Reports (2019)
-
Therapeutic effects of long-circulating miR-135a-containing cationic immunoliposomes against gallbladder carcinoma
Scientific Reports (2017)
-
Poly(ester amine) Composed of Polyethylenimine and Pluronic Enhance Delivery of Antisense Oligonucleotides In Vitro and in Dystrophic mdx Mice
Molecular Therapy - Nucleic Acids (2016)
-
Polyethylenimine-modified Pluronics (PCMs) Improve Morpholino Oligomer Delivery in Cell Culture and Dystrophic mdx Mice
Molecular Therapy (2013)
-
One-year Treatment of Morpholino Antisense Oligomer Improves Skeletal and Cardiac Muscle Functions in Dystrophic mdx Mice
Molecular Therapy (2011)