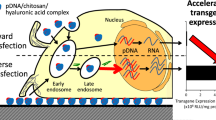
Abstract
The successful gene therapy largely depends on the vector type that allows a selective and efficient gene delivery to target cells with minimal toxicity. Nonviral vectors are much safer and cheaper, can be produced easily in large quantities, and have higher genetic material carrying capacity. However, they are generally less efficient in delivering DNA and initiating gene expression as compared to viral vectors, particularly when used in vivo. As nonviral vectors, polycations may work well for efficient cell uptake and endosomal escape, because they do form compact and smaller complexes with plasmid DNA and carry amine groups, which give positive charge and buffering ability that allows safe escape from endosome/lysosome. However, this is a disadvantage in the following step, which is releasing the plasmid DNA within the cytosol. In order to initiate transcription and enhance gene expression, the polymer/plasmid complex should dissociate after releasing from endosome safely and effectively. There are also other limitations with some of the polycationic carriers, for example, aggregation, toxicity, etc. Intelligent polymers, also called as ‘stimuli responsive polymers’, have a great potential as nonviral vectors to obtain site-, timing-, and duration period-specific gene expression, which is already exhibited in recent studies that are briefly summarized here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Choi YHF et al. In vivo delivery to tumors of DNA complexed with linear poliethylenimine. Hum Gene Ther 1999; 10: 2657–2665.
Bielinska AU, Kukowskalatallo JF, Baker JR . Regulation of gene expression with double-stranded phosphothioate oligoncleotides. Biochim Biophys Acta 1997; 1353: 180–190.
Boussif O, Zanta MA, Behr JP . Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Therapy 1996; 3: 1074–1080.
Erbacher P et al. Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res 1998; 15: 1332–1339.
Ferrari S et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Therapy 1997; 4: 1100–1106.
Smith LC et al. Humoral and cellular immune responses of dogs immunized with a nucleic acid vaccine encoding human carcinoembryonic antigen. Adv Drug Deliv Rev 1998; 30: 115–131.
Godbey WT, Wu KK, Mikos AG . Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci USA 1999; 96: 5177–5181.
Ogris M et al. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy 1999; 6: 4595–4605.
Kwoh DY et al. Stabilization of poly-L-lysine /DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta 1999; 1444: 171–190.
Richardson SCW, Kolbe HVJ, Duncan R . Potential of low molecular mass chitosan as a DNA delivery system: biocompatibility, body distribution and ability to complex and protect DNA. Int J Pharm 1999; 178: 231–243.
Fischer D et al. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res 1999; 16: 1273–1279.
Hwang S, Bellocq N, Davis ME . Effects of structure of β-cyclodextrin-containing polymers on gene delivery. Bioconjugate Chem 2001; 12: 280–290.
Yokoyama M . Gene delivery using temperature responsive polymeric carriers. Drug Discovery Today 2002; 7: 426–432.
Hoffman AS . Bioconjugates of intelligent polymers and recognition proteins for use in diagnostics and affinity separations. Clin Chem 2000; 46: 1478–1486.
Shimoboji T et al. Photoresponsive polymer-enzyme switches. Proc Natl Acad Sci USA 2002; 99: 16592–16596.
Hinrichs WLJ et al. Thermosensitive polymers as carriers for DNA delivery. J Control Release 1999; 60: 249–259.
Kurisawa M, Yokoyama M, Okano T . Gene expression control by temperature with thermo-responsive polymeric gene carriers. J Control Release 2000; 69: 127–137.
Yokoyama M, Kurisawa M, Okano T . Influential factors on temperature-controlled gene expression using thermoresponsive polymeric gene carriers. J Artif Organs 2001; 4: 138–145.
Twaites B et al. Thermo and pH responsive polymers as gene delivery vectors:effect of polymer architecture on DNA complexation in vitro. J Control Release 2004; 97: 551–566.
Nagasaki T et al. Synthesis of a novel water-soluble poliazobenzene dendrimer and photoregulation of affinity toward DNA. Mol Cryst Liq Cryst 2000; 345: 227–232.
Nagasaki T, Wada K, Tamagaki S . Photo-enhancement of transfection efficiency with a novel azobenzene-based cationic lipid. Chem Lett 2003; 32: 88–89.
Berg K et al. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res 1999; 59: 1180–1183.
Hogset A et al. Photochemical transfection: a new technology for light-induced, site-directed gene delivery. Hum Gene Ther 2000; 11: 869–880.
Prasmickaite L et al. Role of endosomes in gene transfection mediated by photochemical internalisation(PCI). J Gene Med 2000; 2: 477–488.
Prasmickaite L, Hogset A, Berg K . Evaluation of different photosensitizers for use in photochemical gene transfection. Photochem Photobiol 2001; 73: 388–395.
Hogset A et al. Light-induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy. Cancer Gene Ther 2002; 9: 365–371.
Brunner S et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Therapy 2000; 7: 401–407.
Prasmickaitea L, Hogset A, Berg K . The role of the cell cycle on the efficiency of photochemical gene transfection. Biochim Biophys Acta 2002; 1570: 210–218.
Dinçer S, Tuncel A, Piskin E . Potential gene delivery vector: N-isopropylacrylamide-ethyleneimine block copolymers. Macromol Chem Phys 2002; 203: 1460–1465.
Türk M et al. In vitro transfection of HeLa cells with temperature sensitive polycationic copolymers. J Control Release 2004; 96: 325–340.
Türk M, Dinçer S, Pişkin E . In vitro transfection of smooth muscle cells with smart polycationic macromolecules. J Biomat Sci: Polym Ed, submitted, 2005.
Acknowledgements
Professor Erhan Pişkin is supported by the Turkish Academy of Sciences as a full member.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dinçer, S., Türk, M. & Pişkin, E. Intelligent polymers as nonviral vectors. Gene Ther 12 (Suppl 1), S139–S145 (2005). https://doi.org/10.1038/sj.gt.3302628
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302628
Keywords
This article is cited by
-
Gene-activated matrix/bone marrow-derived mesenchymal stem cells constructs regenerate sweat glands-like structure in vivo
Scientific Reports (2017)
-
An influenza virus-inspired polymer system for the timed release of siRNA
Nature Communications (2013)
-
Non-viral gene delivery strategies for gene therapy: a “ménage à trois” among nucleic acids, materials, and the biological environment
Journal of Nanoparticle Research (2013)
-
A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells
Cytotechnology (2012)
-
Selective gene delivery to cancer cells secreting matrix metalloproteinases using a gelatin/polyethylenimine/DNA complex
Biotechnology and Bioprocess Engineering (2012)