Abstract
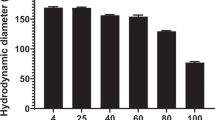
In this study, we investigated to what extent the stability and transduction capacity of polyplexed DNA can be improved by optimizing the condensing peptide sequence. We have synthesized a small library of cationic peptides, at which the lysine/arginine ratio and the cation charge were varied. All peptides were able to compact DNA, at which polyplexes of short lysine-rich sequences were considerably larger than those of elongated or arginine-rich peptides (GM102 and GM202). In addition, the arginine-rich peptides GM102 and GM202 rendered the polyplexes resistant to plasma incubation or DNase I-mediated digestion. While all peptides were found to improve the transfection efficiency in HepG2 cells, only the GM102- and GM202-derived polyplexes could be specifically targeted to HepG2 cells by incorporation of a ligand-derivatized YKAK8WK peptide. We propose that GM102 and GM202 combine the advantage of small condensing peptides to give small-sized polyplexes with the superior stability of condensing polymers, which makes GM102 and GM202 excellent candidates for future in vivo gene therapy studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Li B et al. Lyophilization of cationic lipid–protamine–DNA (LPD) complexes. J Pharm Sci 2000; 89: 355–364.
Mahato RI . Non-viral peptide-based approaches to gene delivery. J Drug Target 1999; 7: 249–268.
Young JL, Dean DA . Nonviral gene transfer strategies for the vasculature. Microcirculation 2002; 9: 35–49.
Wolschek MF et al. Specific systemic nonviral gene delivery to human hepatocellular carcinoma xenografts in SCID mice. Hepatology 2002; 36: 1106–1114.
Armeanu S et al. Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Mol Ther 2000; 1: 366–375.
Domashenko A, Gupta S, Cotsarelis G . Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat Biotechnol 2000; 18: 420–423.
Felgner PL et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA 1987; 84: 7413–7417.
Mahato RI, Kawabata K, Takakura Y, Hashida M . In vivo disposition characteristics of plasmid DNA complexed with cationic liposomes. J Drug Target 1995; 3: 149–157.
Wu CH, Wilson JM, Wu GY . Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem 1989; 264: 16985–16987.
Wilson JM et al. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci USA 1988; 85: 3014–3018.
Wagner E et al. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin–polylysine–DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci USA 1992; 89: 7934–7938.
Gottschalk S et al. A novel DNA–peptide complex for efficient gene transfer and expression in mammalian cells. Gene Therapy 1996; 3: 48–57.
Van Rossenberg SM et al. Targeted lysosome disruptive elements for improvement of parenchymal liver cell specific gene delivery. J Biol Chem 2002; 277: 45803–45810.
Navarro-Quiroga I et al. Improved neurotensin-vector-mediated gene transfer by the coupling of hemagglutinin HA2 fusogenic peptide and Vp1 SV40 nuclear localization signal. Brain Res Mol Brain Res 2002; 105: 86–97.
Merwin JR et al. Targeted delivery of DNA using YEE(GalNAcAH)3, a synthetic glycopeptide ligand for the asialoglycoprotein receptor. Bioconjug Chem 1994; 5: 612–620.
Schaffer DV, Lauffenburger DA . Targeted synthetic gene delivery vectors. Curr Opin Mol Ther 2000; 2: 155–161.
Kircheis R et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Therapy 1997; 4: 409–418.
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Guillem VM et al. Targeted oligonucleotide delivery in human lymphoma cell lines using a polyethyleneimine based immunopolyplex. J Control Release 2002; 83: 133–146.
Haensler J, Szoka Jr FC . Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem 1993; 4: 372–379.
Plank C, Mechtler K, Szoka Jr FC, Wagner E . Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther 1996; 7: 1437–1446.
Sparrow JT et al. Synthetic peptide-based DNA complexes for nonviral gene delivery. Adv Drug Deliv Rev 1998; 30: 115–131.
McKenzie DL, Collard WT, Rice KG . Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res 1999; 54: 311–318.
Wadhwa MS et al. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug Chem 1997; 8: 81–88.
Niidome T et al. Gene transfer into hepatoma cells mediated by galactose-modified alpha-helical peptides. Biomaterials 2000; 21: 1811–1819.
Wadhwa MS, Knoell DL, Young AP, Rice KG . Targeted gene delivery with a low molecular weight glycopeptide carrier. Bioconjug Chem 1995; 6: 283–291.
Zou Y, Zong G, Ling YH, Perez-Soler R . Development of cationic liposome formulations for intratracheal gene therapy of early lung cancer. Cancer Gene Ther 2000; 7: 683–696.
Wolfert MA, Seymour LW . Atomic force microscopic analysis of the influence of the molecular weight of poly(L)lysine on the size of polyelectrolyte complexes formed with DNA. Gene Therapy 1996; 3: 269–273.
Lacey Jr JC, Pruitt KM . Drug–biomolecule interactions: interactions of mononucleotides and polybasic amino acids. J Pharm Sci 1975; 64: 473–477.
Plank C, Tang MX, Wolfe AR, Szoka Jr FC . Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther 1999; 10: 319–332.
Dash PR et al. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Therapy 1999; 6: 643–650.
Kalderon D, Roberts BL, Richardson WD, Smith AE . A short amino acid sequence able to specify nuclear location. Cell 1984; 39: 499–509.
Lanford RE, Kanda P, Kennedy RC . Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell 1986; 46: 575–582.
Perales JC et al. Biochemical and functional characterization of DNA complexes capable of targeting genes to hepatocytes via the asialoglycoprotein receptor. J Biol Chem 1997; 272: 7398–7407.
Chiou HC et al. Enhanced resistance to nuclease degradation of nucleic acids complexed to asialoglycoprotein–polylysine carriers. Nucleic Acids Res 1994; 22: 5439–5446.
Cristiano RJ, Smith LC, Woo SL . Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci USA 1993; 90: 2122–2126.
Oh YK et al. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Therapy 2002; 9: 1627–1632.
Gausepohl H, Boulin C, Kraft M, Frank RW . Automated multiple peptide synthesis. Pept Res 1992; 5: 315–320.
Sliedregt LA et al. Design and synthesis of a multivalent homing device for targeting to murine CD22. Bioorg Med Chem 2001; 9: 85–97.
Commerford SL . In vitro iodination of nucleic acids. Methods Enzymol 1980; 70: 247–252.
Cotten M, Wagner E, Birnstiel ML . Receptor-mediated transport of DNA into eukaryotic cells. Methods Enzymol 1993; 217: 217618–217644.
Acknowledgements
This study was supported by the Netherlands Foundation for Scientific Research (NWO, project 901-01-096), the Netherlands Heart Foundation (project M93 001) and the Chemical Sciences/Foundation of Technical Sciences (CW/STW, project 349-4779).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Rossenberg, S., van Keulen, A., Drijfhout, JW. et al. Stable polyplexes based on arginine-containing oligopeptides for in vivo gene delivery. Gene Ther 11, 457–464 (2004). https://doi.org/10.1038/sj.gt.3302183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302183