Abstract
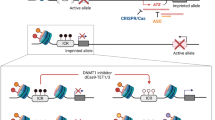
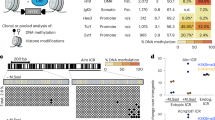
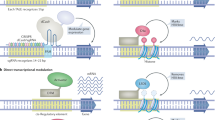
Epigenetic control of transcription is essential for mammalian development and its deregulation causes human disease. For example, loss of proper imprinting control at the IGF2–H19 domain is a hallmark of cancer and Beckwith–Wiedemann syndrome, with no targeted therapeutic approaches available. To address this deficiency, we engineered zinc-finger transcription proteins (ZFPs) that specifically activate or repress the IGF2 and H19 genes in a domain-dependent manner. Importantly, we used these ZFPs successfully to reactivate the transcriptionally silent IGF2 and H19 alleles, thus overriding the natural mechanism of imprinting and validating an entirely novel avenue for ‘transcription therapy’ of human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feinberg A, Vogelstein B . Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983; 301: 89–92.
Robertson K . DNA methylation, methyltransferases, and cancer. Oncogene 2001; 20, 3139–3155.
Ohlsson R et al. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith–Weidemann syndrome. Nature Genet 1993; 4: 94–97.
Giannoukakis N, Deal C, Paquette J, Goodyer C, Polychronakos C . Parental genomic imprinting of the human IGF2 gene. Nature Genet 1993; 4: 98–101.
Zhang Y, Tycko B . Monoallelic expression of the human H19 gene. Nature Genet 1992; 1: 40–44.
Rainier S et al. Relaxation of imprinted genes in human cancer. Nature 1993; 362: 747–749.
Ogawa O et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature 1993; 362: 749–751.
Weksberg R, Shen DR, Fei YL, Song QL, Squire J . Disruption of insulin-like growth factor 2 imprinting in Beckwith–Wiedemann syndrome. Nature Genet 1993; 5: 143–150.
Sun FL, Dean WL, Kelsey G, Allen ND, Reik W . Transactivation of Igf2 in a mouse model of Beckwith–Wiedemann syndrome. Nature 1997; 389: 809–815.
Eversole-Cire P et al. Activation of an imprinted Igf 2 gene in mouse somatic cell cultures. Mol Cell Biol 1993; 13: 4928–4938.
Pabo CO, Peisach E, Grant RA . Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem 2001; 70: 313–340.
Liu Q, Xia Z, Case CC . Validated zinc finger protein designs for all 16 GNN DNA triplet targets. J Biol Chem 2002; 277: 3850–3856.
Dreier B, Beerli RR, Segal DJ, Flippin JD, Barbas CF 3rd . Development of zinc finger domains for recognition of the 5′-ANN-3′ family of DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 2001; 276: 29466–29478.
Segal DJ, Dreier B, Beerli RR, Barbas CF 3rd . Toward controlling gene expression at will: selection and design of zinc finger domains recognizing each of the 5′-GNN-3′ DNA target sequences. Proc Natl Acad Sci USA 1999; 96: 2758–2763.
Rebar EJ, Greisman HA, Pabo CO . Phage display methods for selecting zinc finger proteins with novel DNA-binding specificities. Methods Enzymol 1996; 267: 129–149.
Rebar EJ, Pabo CO . Zinc finger phage: affinity selection of fingers with new DNA-binding specificities. Science 1994; 263: 671–673.
Beerli RR, Segal DJ, Dreier B, Barbas CF 3rd . Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci USA 1998; 95: 14628–14633.
Zhang L et al. Synthetic zinc finger transcription factor action at an endogenous chromosomal site. Activation of the human erythropoietin gene. J Biol Chem 2000; 275: 33850–33860.
Liu PQ et al. Regulation of an endogenous locus using a panel of designed zinc finger proteins targeted to accessible chromatin regions. Activation of vascular endothelial growth factor A. J Biol Chem 2001; 276: 11323–11334.
Beerli RR, Dreier B, Barbas CF 3rd . Positive and negative regulation of endogenous genes by designed transcription factors. Proc Natl Acad Sci USA 2000; 97: 1495–1500.
Bartsevich VV, Juliano RL . Regulation of the MDR1 gene by transcriptional repressors selected using peptide combinatorial libraries. Mol Pharmacol 2000; 58: 1–10.
Xu D, Ye D, Fisher M, Juliano RL . Selective inhibition of P-glycoprotein expression in multidrug-resistant tumor cells by a designed transcriptional regulator. J Pharmacol Exp Ther 2002; 302: 963–971.
Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS . PPAR gamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev 2002; 16: 27–32.
Ekström TJ, Cui H, Li X, Ohlsson R . Promoter-specific IGF2 imprinting status and its plasticity during human liver development. Development 1995; 121: 309–316.
van Dijk MA, van Schaik FM, Bootsma HJ, Holthuizen JS . Initial charaterization of the four promoters of the human IGF II gene. Mol Cell Endocrinol 1991; 81: 81–94.
Vu TH, Hoffman AR . Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature 1994; 371: 714–717.
Hao Y, Crenshaw T, Moulton T, Newcomb E, Tycko B . Tumour-suppressor activity of H19 RNA. Nature 1993; 365: 764–767.
Cui H et al. Inactivation of H19, an imprinted and putative tumor repressor gene, is a preneoplastic event during Wilms' tumorigenesis. Cancer Res 1997; 57: 4469–4473.
Sap J, Munoz A, Schmitt J, Stunnenberg H, Vennstrom B . Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature 1989; 340: 242–244.
Damm K, Thompson CC, Evans RM . Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature 1989; 339: 593–597.
Zenke M, Munoz A, Sap J, Vennstrom B, Beug H . v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell 1990; 61: 1035–1049.
Yao F, Schaffer PA . An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol 1995; 69: 6249–6258.
Koziczak M, Muller H, Helin K, Nagamine Y . E2F1-mediated transcriptional inhibition of the plasminogen activator inhibitor type 1 gene. Eur J Biochem 2001; 268: 4969–4978.
Kiess W, Paquette J, Koepf G, Wolf E, Deal C . Proinsulin-like growth factor-II overexpression does not alter monoallelic H19 gene expression in transfected human embryonic kidney fibroblasts. Biochem Biophys Res Commun 1999; 255: 226–230.
Kugoh H et al. Mouse A9 cells containing single human chromosomes for analysis of genomic imprinting. DNA Res 1999; 6: 165–172.
Inoue J et al. Construction of 700 human/mouse A9 monochromosomal hybrids and analysis of imprinted genes on human chromosome 6. J Hum Genet 2001; 46: 137–145.
Thorvaldsen JL, Duran KL, Bartolomei MS . Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev 1998; 12: 3693–3702.
Ohlsson R, Renkawitz R, Lobanenkov V . CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet 2001; 17: 520–527.
Kanduri C et al. Functional interaction of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 2000; 10: 853–856.
Bell AC, Felsenfeld G . Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 2000; 405: 482–485.
Hark AT et al. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 2000; 405: 486–489.
Kaffer CR et al. A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev. 2000; 14: 1908–1919.
Svensson K et al. The paternal allele of the H19 gene is progressively silenced during early mouse development: the acetylation status of histones may be involved in the generation of variegated expression patterns. Development 1998; 125: 61–69.
Drewell RA, Goddard CJ, Thomas JO, Surani MA . Methylation-dependent silencing at the H19 imprinting control region by MeCP2. Nucleic Acids Res 2002; 30: 1139–1144.
Jouvenot Y, Poirier F, Jami J, Paldi A . Biallelic transcription of Igf2 and H19 in individual cells suggests a post-transcriptional contribution to genomic imprinting. Curr Biol 1999; 9: 1199–1202.
Wu H, Yang WP, Barbas CF 3rd. Building zinc fingers by selection: toward a therapeutic application. Proc Natl Acad Sci USA 1995; 92: 344–348.
Kim JS, Pabo CO . Transcriptional repression by zinc finger peptides. Exploring the potential for applications in gene therapy. J Biol Chem 1997; 272: 29795–29800.
Reik A, Gregory PD, Urnov FD . Biotechnologies and therapeutics: chromatin as a target. Curr Opin Genet Dev 2002; 12: 233–242.
Kim JS, Pabo CO . Getting a handhold on DNA: design of poly-zinc finger proteins with femtomolar dissociation constants. Proc Natl Acad Sci USA 1998; 95: 2812–2817.
Sadowski I, Ma J, Triezenberg S, Ptashne M . GAL4-VP16 is an unusually potent transcriptional activator. Nature 1988; 335: 563–564.
Ruben SM et al. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science 1991; 251: 1490–1493.
Sap J et al. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 1986; 324: 635–640.
Franklin G et al. The human PDGF-B gene is regulated by cell type-specific activator and suppressor elements in the first intron. EMBO J 1991; 10: 1365–1373.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jouvenot, Y., Ginjala, V., Zhang, L. et al. Targeted regulation of imprinted genes by synthetic zinc-finger transcription factors. Gene Ther 10, 513–522 (2003). https://doi.org/10.1038/sj.gt.3301930
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301930
Keywords
This article is cited by
-
Drug discovery with engineered zinc-finger proteins
Nature Reviews Drug Discovery (2003)