Abstract
Application of DNA technology to regulate the transcription of disease-related genes has important therapeutic potential. The transcription factor NFκB plays a pivotal role in the transactivation of inflammatory and adhesion molecule genes, leading to vascular lesion formation. Double-stranded DNA with high affinity for NFκB may be introduced as ‘decoy’ cis elements to bind NFκB and block the activation of genes mediating inflammation, resulting in effective drugs for treating intimal hyperplasia. In this study, we tested the feasibility of NFκB decoy therapy to treat neointimal formation in a porcine coronary artery balloon injury model as a pre-clinical study.
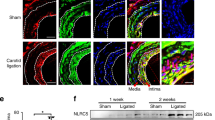
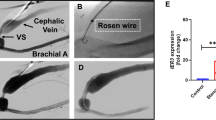
An angioplasty catheter was introduced into the left anterior descending coronary artery of the pig to cause vascular injury. First, we tested the feasibility of transfection of FITC-labeled NFκB decoy ODN using a hydrogel balloon catheter. Fluorescence due to NFκB decoy ODN could be detected throughout the medial layer. Therefore, we transfected NFκB decoy ODN into the balloon-injured LAD using a hydrogel catheter. Histological evaluation demonstrated that the neointimal area in the balloon-injured artery was significantly reduced by NFκB decoy ODN as compared to scrambled decoy ODN at 1 week after single transfection, accompanied by a significant reduction in PCNA-positive stained cells (P<0.01). Interestingly, the reduction of ICAM-positive staining was observed, accompanied by the inhibition of migration of macrophages. Of importance, intravascular ultrasound (IVUS) confirmed that neointimal area in the balloon-injured artery was significantly reduced by NFκB decoy ODN at 4 weeks after transfection (P<0.01). Interestingly, the inhibition of neointimal area was only limited to the lesion transfected with NFκB decoy ODN, while other lesions without NFκB decoy ODN demonstrated a marked increase in neointimal formation.
Here, we report the successful in vivo transfer of NFκB decoy ODN using a hydrogel catheter to inhibit vascular lesion formation in balloon-injured porcine coronary artery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gibbons GH, Dzau VJ . The emerging concept of vascular remodeling. N Engl J Med 1994; 330: 1431–1438.
Lefkovits J, Topol EJ . Pharmacological approaches for the prevention of restenosis after percutaneous coronary intervention. Prog Cardiovasc Dis 1997; 40: 141–158.
Landry DB, Couper LL, Bryant SR, Lindner V . Activation of the NF-κB and I-κB system in smooth muscle cells after rat arterial injury: induction of vascular cell adhesion molecule-1 and monocyte chemotactic protein-1. Am J Pathol 1997; 151: 1085–1095.
Weber C et al. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-κB mobilization and induction of vascular cell adhesion molecule 1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb 1994; 14: 1665.
Ledebur HC, Parks TP . Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. J Biol Chem 1995; 270: 933.
Yasukawa H et al. Inhibition of intimal hyperplasia after balloon injury by antibodies to intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1. Circulation 1997; 95: 1515–1522.
Boucier T, Sukhova G, Libby P . The nuclear factor-κB signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem 1997; 272: 15 817–15 824.
Brand K et al. Activated transcription factor nuclear factor-κB is present in the atherosclerotic lesion. J Clin Invest 1996; 97: 1715–1722.
Landry DB, Couper LL, Bryant SR, Lindner V . Activation of the NF-κB and I-κB system in smooth muscle cells after rat arterial injury: induction of vascular cell adhesion molecule-1 and monocyte chemotactic protein-1. Am J Pathol 1997; 151: 1085–1095.
Cercek B et al. Nuclear factor-κB activity and arterial response to balloon injury. Atherosclerosis 1997; 131: 59–66.
Bellas RE, Lee JS, Sonnenshein GE . Expression of a constitutive NF-κB-like activity is essentioal for proliferation of cultured bovine smooth muscle cells. J Clin Invest 1995; 96: 2521–2527.
Selzman CH et al. Liposomal delivery of purified inhibitory-κBα inhibits tumor necrosis factor-α–induced human vascular smooth muscle proliferation. Circ Res 1999; 84: 867–875.
Erl W et al. Nuclear factor-κB regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ Res 1999; 84: 668–677.
Tomita N, Morishita R, Higaki J, Ogihara T . Strategy for functional inactivation of genes: a novel strategy for gene therapy and gene regulation analysis using transcriptional factor decoy oligonucleotides. Exp Nephrol 1997; 5: 429–434.
Morishita R, Higaki J, Tomita N, Ogihara T . Application of transcription factor ‘decoy’ strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res 1998; 82: 1023–1028.
Morishita R et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-κB binding site prevents myocardial infarction. Nat Med 1997; 3: 894–899.
Sawa Y et al. A novel strategy for myocardial protection using in vivo transfection of cis element ‘decoy’ against NFκB binding site: evidence for a role of NFκB in ischemic-reperfusion injury. Circulation 1997; 96:II-280-285.
Yoshimura S et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element "decoy" of nuclear factor-kB binding site as a novel molecular strategy. Gene Ther 2001;8:1635–1642.
Tomita N et al. In vivo administration of a nulear transcription factor-kappaB decoy suppresses experimental crescentic glomerulonephritis. J Am Soc Nephrol 2000; 11: 1244–1252.
Tomita N et al. Transcription factor decoy for NFκB inhibits TNF-α-induced IL-6 and ICAM-1 expression in endothelial cells. J Hypertens 1998; 16: 993–1000.
Grimm S, Baeuerle PA . The inducible transcription factor NF-κB: structure–function relationship of its protein subunits. Biochem J 1993; 290: 297.
Collins T . Endothelial nuclear factor-κB and the initiation of atherosclerotic lesion. Lab Invest 1993; 68: 499.
Henkel T et al. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-κB subunit. Cell 1992; 68: 1121.
Henkel T et al. Rapid proteolysis of IκBα is necessary for activation of transcription factor NF-κB. Nature 1993; 365: 182–185.
Lawrence R et al. Vascular smooth muscle cells express a constitutive NF-κB-like activity. J Biol Chem 1994;269:28913–28918.
Bellas RE, Lee JS, Sonnenshein GE . Expression of a constitutive NF-κB-like activity is essential for proliferation of cultured bovine smooth muscle cells. J Clin Invest 1995; 96: 2521–2527.
Selzman CH et al. Liposomal delivery of purified inhibitory-κBα inhibits tumor necrosis factor-α–induced human vascular smooth muscle proliferation. Circ Res 1999; 84: 867–875.
Erl W et al. Nuclear factor-κB regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ Res 1999; 84: 668–677.
Cercek B et al. Nuclear factor-κB activity and arterial response to balloon injury. Atherosclerosis 1997; 131: 59–66.
Wadgaonkar R et al. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J Biol Chem 1999; 274: 1879–1882.
Matsumoto K et al. Inhibition of neointima by ACE inhibitor in porcine coronary artery balloon injury model. Hypertension 2001; 37: 270–274.
Morishita R et al. Intimal hyperplasia after vascular injury is inhibited by antisense cdk 2 kinase oligonucleotides. J Clin Invest 1994; 93: 1458–1464.
Ge J et al. Intravascular ultrasound imaging of arterial wall architecture. Echocardiography. 1992; 9: 475–483.
Acknowledgements
This work was partially supported by a Grant-in-Aid from the Ministry of Public Health and Welfare, a Grant-in-Aid for the Development of Innovative Technology, a Grant-in-Aid from Japan Promotion of Science, and Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamasaki, K., Asai, T., Shimizu, M. et al. Inhibition of NFκB activation using cis-element ‘decoy’ of NFκB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther 10, 356–364 (2003). https://doi.org/10.1038/sj.gt.3301875
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301875
Keywords
This article is cited by
-
Targeting N-acetylglucosamine-bearing polymer-coated liposomes to vascular smooth muscle cells
Journal of Artificial Organs (2011)
-
Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice
BMC Cancer (2007)
-
Transcription factor decoy oligonucleotide-based therapeutic strategy for renal disease
Clinical and Experimental Nephrology (2007)
-
Prevention of abdominal aortic aneurysms by simultaneous inhibition of NFκB and ets using chimeric decoy oligonucleotides in a rabbit model
Gene Therapy (2006)