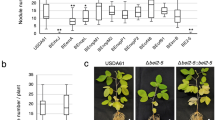
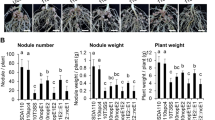
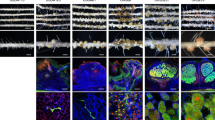
Abstract
The symbiosis between bacteria of the genus Rhizobium and their leguminous host plants results in the formation of root nodules in a species-specific way, in that a particular bacterial species can nodulate only a limited number of host species. In fast-growing Rhizobium species many nodulation (nod) genes, including the functional interchangeable or common nod genes nodA,B,C,I,J and nodD and the host-specificity genes nodE.F, are localized on large Sym (for symbiosis) plasmids1–5. On the Rhizobium meliloti Sym plasmid three nodD genes have been localized. Two of these, nodD1 and nodD2, are required for efficient nodulation of the host plant (ref 6 and M. Honma, personal communication). Exudates of leguminous plants induce the expression of several Sym-plasmid-localized nod operons7–10, a process in which the constitutively expressed nodD product is supposed to act as a positive regulator8,9. The inducing compounds found in these exudates have been identified as flavones, flavanones or closely related compounds11–14. We report here that the nodD products of the various fast-growing Rhizobium species differ from each other in that they confer different responsiveness, in a species-specific way, to different sets of flavonoids and exudates. Moreover, in one case, the nodD gene was shown to be a determinant of host-specific nodulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Kondorosi, E. & Kondorosi, A. Trends biochem. Sci. 11, 296–299 (1986). 2. Shearman, C. A., Rossen, L., Johnston, A. W. B. & Downie, J. A. EMBOJ. 5,647–652 (1986). 3. Evans, I. J. & Downie, J. A. Gene 43, 95–101 (1986). 4. Djordjevic, M. C., Innes, R. W., Wijffelman, C. A., Schofield, P. R. & Rolfe, B. G. PI. Molec. Biol. 6, 389–401 (1986). 5. Spaink, H. P., Okker, R. J. H., Wijffelman, C. A., Pees, E. & Lugtenberg, B. J. J. in Recognition in Microbe–Plant Symbiotic and Pathogenic Interactions (ed. Lugtenberg, B. J. J.) 55–68 (Springer, Heidelberg, 1986). 6. Gottfert, M. et al J. molec. Biol. 191, 411–420 (1986). 7. Innes, R. W. et al. Molec. gen. Genet. 201, 426–432 (1985). 8. Mulligan, J. T. & Long, S. R. Proc. natn. Acad. Sci. U.S.A. 82, 6609–6613 (1985). 9. Rossen, L., Shearman, C. A., Johnston, A. W. B. & Downie, J. A. EMBO J. 4, 3369–3373 (1985). 10. Van Brussel, A. A. N. et al. J. Bact. 165, 517–522 (1986). 11. Peters, N. K., Frost, J. & Long, S. Science 233, 977–980 (1986). 12. Redmond, J. W. et al. Nature 323, 632–635 (1986). 13. Firmin, J. L., Wilson, K. E., Rossen, L. & Johnston, A. W. B. Nature 324, 90–94 (1986). 14. Zaat, S. A. J. et al J. Bact. 169, 198–204 (1987). 15. Rossen, L., Johnston, A. W. B. & Downie, J; A. Nucleic Acids Res. 12, 9497–9508, (1984). 16. Egelhoff, T. T., Fisher, R. F., Jacobs, T. W., Mulligan, J. T. & Long, S. R. DNA 4, 241–248 (1985). 17. Schofield, P. R. & Watson, J. M. Nucleic Acids Res. 14, 2891–2903 (1986). 18. Jacobs, T. W., Egelhoff, T. T. & Long, S. R. /. Bact. 162, 469–476 (1985). 19. Spaink, H. P., Okker, R. J. H., Wijffelman, C. A., Pees, E. & Lugtenberg, B. PI. motec. Biol. (in the press). 20. Djordjevic, M. A. et al. PI. molec. Biol. 4, 147–160 (1985). 21. Wijffelman, C. A., Pees, E., Van Brussel, A. A. N., Okker, R. J. H. & Lugtenberg, B. J. J. Arch. Microbiol. 143, 225–232 (1985). 22. Fisher, R. F., Tu, J. K. & Long, S. R. Appl. env. Microbiol. 49, 1432–1435 (1985). 23. Hooykaas, P. J. J., Snijdewint, F. G. M. & Schilperoort, R. A. Plasmid 8, 73–82 (1982). 24. Rostas, K., Kondorosi, E., Horvath, B., Simoncsits, A. & Kondorosi, A. Proc. natn. Acad. Sci. U.S.A. 83, 1757–1761 (1986). 25. Johnston, A. W. B. et al. Nature 276, 635–636 (1978). 26. Winarno, R. & Lie, T. A. Plant. Soil 51, 135–142 (1979). 27. Miller, J. H. Experiments in Molecular Genetics (Cold Spring Harbor Laboratory, New York, 1972). 28. Djordjevic, M. A., Schofield, P. R. & Rolfe, B. G. Molec. gen. Genet. 200, 463–471 (1985). 29. Van Brussel, A. A. N., Tak, T., Wetselaar, A., Pees, E. & Wijffelman, C. A. PL Sci. Lett. 27, 317–325 (1982). 30. Long, S. R, Buikema, W. J. & Ausubel, R. M. Nature 298, 485–488 (1982). 31. Debelle, F. & Sharma, S. B. Nucleic. Acids Res. 14, 7453–7472 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spaink, H., Wijffelman, C., Pees, E. et al. Rhizobium nodulation gene nodD as a determinant of host specificity. Nature 328, 337–340 (1987). https://doi.org/10.1038/328337a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/328337a0
This article is cited by
-
Exploring a rhizobium to fix nitrogen in non-leguminous plants by using a tumor-formation root pathogen
Phytopathology Research (2022)
-
Role of nodD gene product and flavonoid interactions in induction of nodulation genes in Mesorhizobium ciceri
Physiology and Molecular Biology of Plants (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.