Abstract
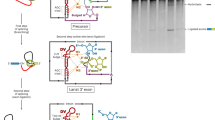
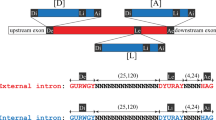
Introns are excised from full-length transcripts (pre-messenger RNAs) of eukaryotic genes in two steps1,2. First, the pre-mRNA is cleaved at the 5′ splice site and a branched (lariat) intermediate is formed1–6. Then, cleavage at the 3′ splice site and ligation of the two exons leads to the release of the lariat intron. The intron sequence which accepts the 5′ end to form the lariat branch is strictly conserved in yeast7–9, but shows more variation in eukary-otes10,11. To investigate the requirements for branch formation in eukaryotes further, we have studied in vitro splicing of a rabbit globin gene intron with mutations of the normal branch-accepting adenosine nucleotide. We conclude that all four nucleotides can serve as branch acceptors, but that A and C are preferred to G and U in lariat formation. Mutation of the normal A to G or U can lead to an A residue one nucleotide upstream of the normal branch site being used instead. Only branches to A or C participate efficiently in the second splicing step.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Padgett, R. A., Konarska, M. M., Grabowski, P. J., Hardy, S. F. & Sharp, P. A. Science 225, 898–903 (1984).
Ruskin, B., Krainer, A. R., Maniatis, T. & Green, M. R. Cell 38, 317–331 (1984).
Zeitlin, S. & Efstratiadis, A. Cell 39, 589–602 (1984).
Domdey, H. et al. Cell 39, 611–621 (1984).
Rodriguez, J. R., Pikielny, C. W. & Rosbash, M. Cell 39, 603–610 (1984).
Konarska, M. M., Grabowski, P. J., Padgett, R. A. & Sharp, P. A. Nature 313, 552–557 (1985).
Langford, C. J. & Gallwitz, D. Cell 33, 519–527 (1983).
Pikielny, C. W., Teem, J. L. & Rosbash, M. Cell 34, 395–403 (1983).
Langford, C. J., Klinz, F.-J., Donath, C. & Gallwitz, D. Cell 36, 645–653 (1984).
Keller, E. B. & Noon, W. A. Proc. natn. Acad. Sci. U.S.A. 81, 7417–7420 (1984).
Reed, R. & Maniatis, T. Cell 41, 119–126 (1985).
Ruskin, B., Greene, J. M. & Green, M. R. Cell 41, 833–844 (1985).
Padgett, R. A. et al. Proc. natn. Acad. Sci. U.S.A. 82, 8349–8353 (1985).
Vab Ooyen, A., van den Berg, J., Mantei, N. & Weissmann, C. Science 206, 337–344 (1979).
Ruskin, B. & Green, M. R. Science 229, 135–140 (1985).
Wallace, J. C. & Edmonds, M. Proc. natn. Acad. sci. U.S.A. 80, 950–954 (1983).
Black, D. L., Chabot, B. & Steitz, J. A. Cell 42, 737–750 (1985).
Ruskin, B. & Green, M. R. Cell 43, 131–142 (1985).
Aebi, M., Hornig, H., Padgett, R. A., Reiser, J. & Weissmann, C. Cell (in the press).
Newman, A. J., Lin, R. J., Cheng, S. C. & Abelson, J. Cell 42, 335–344 (1985).
Jacquier, A. & Rosbash, A. Proc. natn. Acad. sci. U.S.A. 83, 5835–5839 (1986).
Melton, D. A. et al. Nucleic Acids Res. 9, 7035–7056 (1984).
Konarska, M. M., Padgett, R. A. & Sharp, P. A. Cell 38, 731–736 (1984).
Dignam, J. D., Lebovitz, R. M. & Reeder, R. G. Nucleic Acids Res. 11, 1475–1489 (1983).
Maniatis, T., Fritsch, E. F. & Sambrook, J. Molecular Cloning, a Laboratory Manual (Cold Spring Harbor Laboratory, New York, 1982).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad. sci. U.S.A. 74, 5463–5467 (1977).
Heidecker, G., Messing, J. & Gronenborn, B. Gene 10, 69–73 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hornig, H., Aebi, M. & Weissmann, C. Effect of mutations at the lariat branch acceptor site on β-globin pre-mRNA splicing in vitro. Nature 324, 589–591 (1986). https://doi.org/10.1038/324589a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/324589a0
This article is cited by
-
The intronic branch point sequence is under strong evolutionary constraint in the bovine and human genome
Communications Biology (2021)
-
Engineering the splice acceptor for improved gene expression and viral titer in an MLV-based retroviral vector
Gene Therapy (2004)
-
Evidence for two active sites in the spliceosome provided by stereochemistry of pre-mRNA splicing
Nature (1993)
-
Efficiency and specificity of gene isolation by exon amplification
Mammalian Genome (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.