Abstract
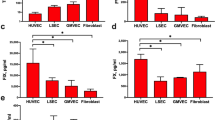
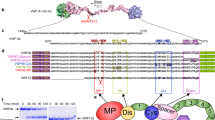
Von Willebrand factor (vWF), a multifunctional haemostatic glycoprotein derived from endothelial cells and megakaryocytes, mediates platelet adhesion to injured subendothelium and binds coagulation factor VIII in the circulation. Native vWF is a disulphide-bonded homopolymer; the monomeric subunits, of apparent relative molecular mass (Mr) 220,000 (220K) are derived from an intracellular precursor estimated at 260–275K (refs 1–5). Multimer assembly is preceded by the formation of dimers, linked near their C-termini, which then assemble into filamentous polymers. The importance of the removal of the large vWF pro-polypeptide during multimer assembly, and whether this or other stages of the complex post-translational processing require components specific to endothelial cells or megakaryocytes, is unknown. Here we report an analysis of the complete sequence of pre-pro-vWF and expression of the molecule in heterologous cells. The vWF precursor is composed of several repeated subdomains. When expressed in COS and CHO cells, it is cleaved and assembled into biologically active high relative molecular mass disulphide bonded multimers. This suggests that the information for assembly of this complex molecule resides largely within its primary structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Legaz, M. E. et al. J. biol. Chem. 248, 3946–3955 (1973).
Shapiro, G. A. et al. J. clin. Invest. 52, 2198–2210 (1973).
Wagner, D. D. & Marder, V. J. J. biol. Chem. 258, 2065–2067 (1983).
Lynch, D.C. et al. J. biol. Chem. 258, 12757–12760 (1983).
Wagner, D. D. & Marder, V. J. J. Cell Biol. 99, 2123–2130 (1984).
Fay, P. J. et al. Science 232, 995–998 (1986).
Perlman, D. & Halvorson, H. O. J. molec. Biol. 167, 391–409 (1983).
Federici, A. B. et al. J. clin. Invest. 74, 2049–2055 (1984).
Sadler, J. E. et al. Proc. natn. Acad. Sci. U.S.A. 82, 6394–6398 (1985).
Shelton-Inloes, B. B., Titani, K. & Sadler, J. E. Biochemistry 25, 3164–3171 (1986).
Counts, R. B., Paskell, S. L. & Elgee, S. K. J. clin. Invest. 62, 702–709 (1978).
Hessel, B. et al. Thromb. Res. 35, 637–651 (1984).
Ohmori, K. et al. J. Cell Biol. 95, 632–640 (1982).
Slayter, H. et al. J. biol. Chem. 260, 8559–8563 (1985).
Fowler, W. E. et al. J. clin. Invest. 76, 1491–1500 (1985).
Mosher, D. F. Prog. Hemost. Thromb. 5, 111–151 (1980).
Doolittle, R. F. Scient. Am. 245, 126–130 (1981).
Pierschbacher, M. D. & Ruoslahti, E. Nature 309, 30–33 (1984).
Pytela, R. et al. Science 231, 1559–1562 (1986).
Ruoslahti, E. & Pierschbacher, M. D. Cell 44, 517–518 (1986).
Kaufman, R. J. et al. Proc. natn. Acad. Sci. U.S.A. 83, 3136–3140 (1986).
Goodman, R. H. et al. J. biol. Chem. 257, 1156–1159 (1982).
Vasicek, T. J. et al. Proc. natn. Acad. Sci. U.S.A. 80, 2127–2131 (1983).
Bell, G. I. et al. Nature 304, 368–371 (1983).
Wiborg, O. et al. Proc. natn. Acad. Sci. U.S.A. 81, 1067–1069 (1984).
Rosenfeld, M. G., Amara, S. G. & Evans, R. M. Science 225, 1315–1320 (1984).
Leiter, A. B. et al. J. biol. Chem. 260, 13013–13017 (1985).
Warren, T. G. & Shields, D. Cell 39, 547–555 (1984).
Bockenstedt, P., Greenberg, J. M. & Handin, R. I. J. clin. Invest. 77, 743–749 (1986).
Bockenstedt, P., McDonagh, J. & Handin, R. I. J. clin. Invest. 78, 551–556 (1986).
Lynch, D. C. et al. Cell 41, 49–56 (1985).
Ginsberg, D. et al. Science 228, 1401–1406 (1985).
Verveij, C. L. et al. Nucleic Acids Res. 13, 4699–4717 (1985).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad Sci. U.S.A. 74, 5463–5467 (1977).
Biggin, M. D., Gibson, T. J. & Hong, G. F. Proc. natn. Aad. Sci. U.S.A. 80, 3963–3965 (1983).
Poncz, M. et al. Proc. natn. Acad. Sci. U.S.A. 79, 4298–4302 (1982).
Dale, R. M. K., McClure, B. A. & Houchins, J. P. Plasmid 13, 31–40 (1985).
Chen, E. Y. & Seeburg, P. H. DNA 4, 165–170 (1985).
Vieira, J. & Messing, J. Gene 19, 259–268 (1982).
Wong, G. G. et al. Science 228, 810–815 (1985).
Orkin, S. H. et al. Molec. cell. Biol. 5, 762–767 (1985).
Sandri-Goldin, R. M. et al. Molec. cell. Biol. 1, 743–752 (1981).
Lopata, M. A., Cleveland, D. W. & Sollner-Webb, B. Nucleic Acids Res. 12, 5707–5717 (1984).
Luthman, H. & Magnusson, G. Nucleic Acids Res. 11, 1295–1308 (1983).
Kaufman, R. J. Proc. natn. Acad. Sci. U.S.A. 82, 689–693 (1985).
Kaufman, R. J. et al. Molec. cell. Biol. 5, 1750–1759 (1985).
Laemmli, U. K. Nature 227, 680–685 (1970).
Bonthron, D. T. et al. Nucleic Acids Res. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonthron, D., Handin, R., Kaufman, R. et al. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature 324, 270–273 (1986). https://doi.org/10.1038/324270a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/324270a0
This article is cited by
-
Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin II/I and vertebrate apolipoprotein B
BMC Evolutionary Biology (2007)
-
The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter
Nature Genetics (1999)
-
Stable expression in Chinese hamster ovary cells of a homogeneous recombinant active fragment of human platelet glycoprotein Ib?
Cytotechnology (1995)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.