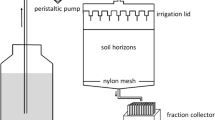
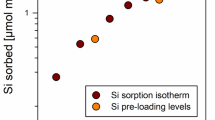
Abstract
Aluminium is a pH-sensitive element that can cause acute toxicity symptoms in some organisms at aqueous activities of 10 µM or less1–3. Scientists working on agricultural systems have long been concerned with the deleterious effects of aluminium on crop roots4,5. More recently, environmental scientists have reported a potentially harmful biogeochemical link between acidic deposition onto forest soils and aluminium toxicity in forest and aquatic communities of northeastern North America and northern Europe6–8. Because of this general interest in aluminium toxicity as an environmental threat, there have been renewed efforts to model the chemistry and transport of aqueous aluminium in soils and surface waters. Here we propose that much of the spatial and temporal variability in aqueous aluminium chemistry can be accounted for by a two-component equilibrium model involving a solid-phase humic adsorbent and an aluminium trihydroxide mineral phase. Inputs for the model are solution pH, copper-extractable organic aluminium and the titratable carboxyl content of soil humus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Haug, A. CRC Crit. Rev. Pl. Sci. 1, 345–373 (1984).
Baker, J. P. & Schofield, C. L. Wat. Air, Soil Pollut. 18, 289–309 (1982).
Hue, N. V., Craddock, G. R. & Adams, F. Soil Sci. Soc. Am. J. 50, 28–34 (1986).
Hardy, F. J. agric. Sci. 16, 616–630 (1926).
Pavan, M. A. & Bingham, F. T. Soil Sci. Soc. Am. J. 46, 993–997 (1982).
Cronan, C. S. & Schofield, C. L. Science 204, 304–306 (1979).
Ulrich, B., Mayer, R. & Khanna, P. K. Soil Sci. 130, 193–199 (1980).
Henriksen, A., Skogheim, O. K. & Rosseland, B. O. Vatten 2, 255–260 (1984).
Christophersen, N., Seip, H. M. & Wright, R. F. Wat. Resour. Res. 18, 977–996 (1982).
Nordstrom, D. K. & Ball, J. W. Science 232, 54–56 (1986).
Bloom, P. R., McBride, M. B. & Weaver, R. M. Soil Sci. Soc. Am. J. 43, 488–493 (1979).
Cronan, C. S. Oikos 34, 272–281 (1980).
Driscoll, C. T., Van Breemen, N. & Mulder, J. Soil Sci. Soc. Am. J. 49, 437–444 (1985).
Hooper, R. P. & Shoemaker, C. A. Science 229, 463–465 (1985).
Soil Survey Staff Soil Taxonomy. (Soil Conservation Service, USDA Washington D.C., 1981).
Mattigod, S. V. & Sposito, G. Am. chem. Soc. Symp. Ser. 93, 837–856 (1979).
Nordstrom, D. K., Valentine, S. D., Ball, J. W., Plummer, L. N. & Jones, B. F. U.S.G.S. Water-Resources Investigation Report 84–4186 (1984).
Johnson, N. M., Driscoll, C. T., Eaton, J. S., Likens, G. E. & McDowell, W. H. Geochim. cosmochim. Acta 45, 1421–1437 (1981).
Bloom, P. R., McBride, M. B. & Chadbourne, B. Soil Sci. Soc. Am. J. 41, 1068–1073 (1977).
Langmuir, D. in Adsorption from Aqueous Solutions (ed. Tewai, P. H.) 1–18 (Plenum, New York, 1981).
Hargrove, W. L. & Thomas, G. W. Soil Sci. Soc. Am. J. 48, 1458–1460 (1984).
Driscoll, C. T. Int. J. envir. analyt. Chem. 16, 267–283 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cronan, C., Walker, W. & Bloom, P. Predicting aqueous aluminium concentrations in natural waters. Nature 324, 140–143 (1986). https://doi.org/10.1038/324140a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/324140a0
This article is cited by
-
A novel pyrazoline-based turn-on fluorescent probe for highly sensitive detection of Al3+ in aqueous solution and its applications
Chemical Papers (2024)
-
Fabrication of a New Coumarin Based Fluorescent “turn-on” Probe for Distinct and Sequential Recognition of Al3+ and F− Along With Its Application in Live Cell Imaging
Journal of Fluorescence (2023)
-
Synthesis and Characterization of a Carbazole-Based Schiff Base Capable of Detection of Al3+ in Organic/Aqueous Media
Journal of Fluorescence (2022)
-
A Comprehensive Review on Thiophene Based Chemosensors
Journal of Fluorescence (2022)
-
The Effect of Natural Geochemical Background on Neurological and Mental Health
Exposure and Health (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.