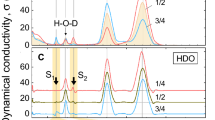
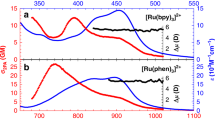
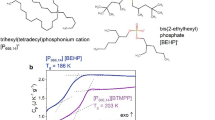
Abstract
Solution studies of halide complexes of the transition metals, of general type [MXn]y− (X=Cl or Br), have been severely hampered by the existence of both solvation and solvolysis phenomena1. We demonstrate here that the use of room-temperature ionic liquids (often misleadingly referred to as molten salts) as solvents circumvents both of these problems, and permits the first reliable solution spectra to be recorded for these species; in some cases the spectra exhibit quite remarkable resolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lever, A. B. P. Inorganic Electronic Spectroscopy 2nd edn (Elsevier, Amsterdam, 1984).
Seddon, E. A. & Seddon, K. R. The Chemistry of Ruthenium (Elsevier, Amsterdam, 1984).
Jørgensen, C. K. Inorganic Complexes (Academic, London, 1963).
Gruen, D. M. & McBeth, R. L. Pure appl. Chem. 6, 23–47 (1963).
Hurley, F. H. & Wier, T. P. J. electrochem. Soc. 98, 203–206 (1951).
Laher, T. M. & Hussey, C. L. Inorg. Chem. 20, 4201–4206 (1981).
Wilkes, J. S., Levisky, J. A., Wilson, R. A. & Hussey, C. L. Inorg. Chem. 21, 1263–1264 (1982).
Sanders, J. R., Ward, E. H. & Hussey, C. L. J. electrochem. Soc. 133, 325–330 (1986).
Hussey, C. L. Adv. molten Salt Chem. 5, 185–229 (1983).
Jørgensen, C. K. Molec. Phys. 2, 309–332 (1959).
Clark, R. J. H. & Turtle, P. C. Chem. Phys. Lett. 51, 265–268 (1977); JCS Faraday Trans. II 74, 2063–2076 (1978).
Allen, G. C., Al Mobarak, R., El-Sharkawy, G. A. M. & Warren, K. D. Inorg. Chem. 11, 787–796 (1972).
Ikeda, K.-I. & Maeda, S. Inorg. Chem. 17, 2698–2701 (1978).
Appleby, D. et al. JCS chem. Commun., 483–485 (1986).
Clark, R. J. H. & Dines, T. J., Molec. Phys. 52, 859–870 (1984).
Collingwood, J. C., Schatz, P. N. & McCarthy, P. J. Molec. Phys. 30, 469–491 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Appleby, D., Hussey, C., Seddon, K. et al. Room-temperature ionic liquids as solvents for electronic absorption spectroscopy of halide complexes. Nature 323, 614–616 (1986). https://doi.org/10.1038/323614a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/323614a0
This article is cited by
-
Nanoporous Polymer Films of Cyanate Ester Resins Designed by Using Ionic Liquids as Porogens
Nanoscale Research Letters (2017)
-
Fluorescent Probe Studies of Polarity and Solvation within Room Temperature Ionic Liquids: A Review
Journal of Fluorescence (2012)
-
Thermodynamic investigation of room temperature ionic liquid
Journal of Thermal Analysis and Calorimetry (2007)
-
Thermodynamic investigation of room temperature ionic liquid
Journal of Thermal Analysis and Calorimetry (2006)
-
Applications of functionalized ionic liquids
Science in China Series B: Chemistry (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.