Abstract
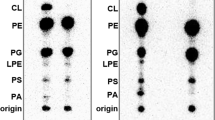
The LamB protein is an integral membrane protein of the outer membrane of Escherichia coli. We have now found that, when synthesized in an E. coli cell-free translation system supplemented with inverted vesicles derived from the E. coli inner membrane, LamB protein is integrated into the vesicle membrane as assayed by its resistance to extraction at alkaline pH. These data suggest that the inner membrane is the primary site for integration of LamB protein prior to subsequent sorting to the outer membrane. When synthesized in a wheat germ cell-free translation system supplemented with canine microsomal membranes, LamB protein is glycosylated at one or two cryptic sites, and surprisingly, it is translocated across instead of being integrated into the vesicle membrane. We suggest that the translocation machinery of the microsomal membrane, although able to recognize the signal sequences) of LamB, is unable to recognize its stop-transfer sequences), thereby yielding translocation instead of integration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blobel, G. Proc. natn. Acad. Sci. U.S.A. 77, 1496–1500 (1980).
Nikaido, H. & Vaara, M. Microbiol. Rev. 49, 1–32 (1985).
Rosenbusch, J. P. J. biol. Chem. 249, 8019–8029 (1974).
Ishii, J. N., Okajima, Y. & Nakee, T. FEBS Lett. 134, 217–220 (1981).
Palva, E. T. & Westermann, P. FEBS Lett. 99, 77–80 (1979).
Nakae, T. & Ishii, J. N. Annls Microbiol. Inst. Pasteur, Paris 133A, 21–25 (1982).
Dorset, D. L., Engel, A., Massalski, A. & Rosenbusch, J. P. Biophys. J. 45, 128–,129 (1984).
Boehler-Kohler, B. A., Boos, W., Dieterle, R. & Benz, R. J. Bact. 138, 33–39 (1979).
Schindler, H. & Rosenbusch, J. P. Proc. natn. Acad. Sci. U.S.A. 78, 2302–2306 (1981).
Luckey, M. & Nikaido, H. Proc. natn. Acad. Sci. U.S.A. 77, 167–171 (1980).
Ferenci, T. & Boos, W. J. J. supramolec. Struc. 13, 101–116 (1980).
Neuhaus, J. M., Schindler, H. & Rosenbusch, J. P. EMBO J. 2, 1987–1991 (1983).
Wandersman, C., Schwartz, M. & Ferenci, T. J. Bact. 140, 1–13 (1977).
Bavoil, P. & Nikaido, H. J. biol. Chem. 256, 11385–11388 (1981).
Randall‐Hazelbauer, L. & Schwartz, M. J. Bact. 116, 1436–1446 (1973).
Clement, J. M. & Hofnung, M. Cell 27, 507–514 (1981).
Müller, M. & Blobel, G. Proc. natn. Acad. Sci. U.S.A. 81, 7421–7425 (1984).
Marchal, C., Perrin, D., Hedgpeth, J. & Hofnung, M. Proc. natn. Acad. Sci. U.S.A 77, 1491–1495 (1980).
Krieg, P. A. & Melton, D. A. Nucleic Acids Res. 12, 7057–7070 (1984).
Schnaitman, C. A. J. Bact. 104, 890–901 (1970).
Steck, T. L. & Yu, J. J. supramolec. Struct. 1, 220–248 (1973).
Fujiki, Y., Hubbard, A. L., Fowler, S. & Lazarow, P. B. J. Cell Biol. 93, 97–102 (1982).
Gilmore, R. & Blobel, G. Cell 42, 497–505 (1985).
Smit, J. & Nikaido, H. J. Bact. 135, 687–702 (1978).
Bayer, M. H. in Membrane Biogenesis (ed. Tzagaloff, A.) 383–437 (Plenum, New York, 1975).
Bayer, M. H., Costello, G. P. & Bayer, M. E. J. Bact. 149, 758–767 (1982).
Ishidate, K. et al. 261, 428–443 (1986).
Pugsley, A. P. & Schwartz, M. FEMS Microbiol. Rev. 32, 3–38 (1985).
Erickson, A. H. & Blobel, G. Meth. Enzym. 96, 38–49 (1983).
Walter, P. & Blobel, G. Proc. natn. Acad. Sci U.S.A. 77, 7112–7116 (1980).
Müller, M., Ibrahimi, I., Chang, C. N., Walter, P. & Blobel, G. J. biol. Chem. 257, 11860–11863 (1982).
Walter, P. & Blobel, G. Meth. Enzym. 96, 682–691 (1983).
Walter, P. & Blobel, G. Meth. Enzym. 96, 84–93 (1983).
Walter, P. & Blobel, G. J. Cell Biol. 91, 557–561 (1981).
Erickson, A. H. & Blobel, G. J. biol. Chem. 254, 11771–11774 (1979).
Friedlander, M. & Blobel, G. Nature 318, 338–343 (1985).
Van Heijne, G. & Blomberg, C. Eur. J. Biochem. 97, 175–181 (1979).
Engelman, D. M. & Steitz, T. A. Cell 23, 411–422 (1981).
Mizushima, S. & Yamada, H. Biochim. biophys. Acta 375, 44–53 (1975).
Dewey, M. M. & Barr, B. Curr. Topics Membrane Transport 1, 6 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watanabe, M., Hunt, J. & Blobel, G. In vitro synthesized bacterial outer membrane protein is integrated into bacterial inner membranes but translocated across microsomal membranes. Nature 323, 71–73 (1986). https://doi.org/10.1038/323071a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/323071a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.