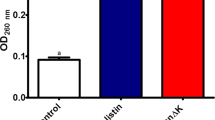
Abstract
Several laboratories, including our own, have reported the synthesis and activity of certain low relative molecular mass inhibitors of mammalian serine proteases1–4, especially human leukocyte elastase (HLE, EC 3.4.21.37), an enzyme whose degradative activity on lung elastin has been implicated as a major causative factor in the induction of pulmonary emphysema5, and which is present in the azurophil granules of human polymorphonuclear leukocytes (PMN). Normally, these granules fuse with phagosomes containing engulfed foreign material (such as bacteria), and HLE, in combination with other lysosomal enzymes, catabolizes the particles6,7. Under certain pathological conditions, however, PMN become attached to host protein (elastin fibres, basement membrane, connective tissue, immune complexes)8,9, and in response to this adherence, the granules may fuse with the PMN outer membrane and release their contents, including HLE, directly onto the tissue10. Besides emphysema, HLE may also contribute to the pathogenesis of disease states such as adult respiratory distress syndrome11, and its potential involvement in rheumatoid arthritis12 makes HLE inhibitors of considerable interest. It is known that cephalosporin antibiotics (for example, cephalothin (compound I, Table 2)) are acylating inhibitors of bacterial serine proteases which help synthesize the cell wall by performing a transpeptidation reaction on a peptidyl substrate bearing a D-Ala-D-Ala terminus13. We now report that neutral cephalosporins (that is, compounds not bearing a free carboxyl at position C-4) can be modified to become potent time-dependent inhibitors of HLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Powers, J. C. Am. Rev. respir. Dis. 127, S54–S58 (1983).
Zimmerman, M. et al. J. biol Chem. 255, 9848–9851 (1980).
Ashe, B. M., Clark, R. L., Jones, H. & Zimmerman, M. J. biol. Chem. 256, 11603–11606 (1981).
Moorman, A. R. & Abeles, R. J. J. Am. chem. Soc. 104, 6785–6786 (1982).
Mittman, C. (ed.) Pulmonary Emphysema and Proteolysis 1–537 (Academic, New York, 1972).
Thorne, K. J. I., Oliver, R. C. & Barrett, A. J. Infect. Immun. 14, 555–563 (1976).
Blondin, J. & Janoff, A. J. clin. Invest. 58, 971–979 (1976).
Starkey, P. M. & Barrett, A. Biochem. J. 155, 265–271 (1976).
Schmidt, W. & Havemann, K. Hoppe-Seylers Z. physiol. Chem. 355, 1077–1082 (1974).
Henson, P. M. J. Immun. 107, 1547–1557 (1971).
Sprung, C. L., Schultz, D. R. & Clerch, A. R. New Engl. J. Med. 304, 1301–1302 (1984).
Velvart, M. Rheumatol. Int. 1, 121–130 (1981).
Ghuysen, J.-M. in The Bacterial D-D Carboxypeptides/transpeptidase Enzyme System, 115–143 (University of Tokyo Press, 1976).
Schechter, I. & Berger, A. Biochem. biophys. Res. Commun. 27, 157–162 (1967).
Zimmerman, M. Colloquium Ges. biolog. Chem. 30, 186–195 (1979).
Brenner, D. G. & Knowles, J. R. Biochemistry 23, 5833–5839 (1984).
Brenner, D. G. & Knowles, J. R. Biochemistry 23, 5839–5846 (1984).
Stetson, C. A. Jr J. exp. Med. 94, 347–358 (1951).
Thomas, L. in The Inflammatory Process 456–459 (Academic, New York, 1965).
Stetson, C. A. & Good, R. A. J. exp. Med. 93, 49–64 (1951).
Argenbright, L. W., Ashe, B. M., Zimmerman, M. & Bonney, R. J. Fedn Proc. 43, 405 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doherty, J., Ashe, B., Argenbright, L. et al. Cephalosporin antibiotics can be modified to inhibit human leukocyte elastase. Nature 322, 192–194 (1986). https://doi.org/10.1038/322192a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/322192a0
This article is cited by
-
Synthesis and anticancer properties of 7α-chloro-3-methyl-1,1-dioxoceph-3-em-4-carboxylic acid esters
Chemistry of Heterocyclic Compounds (2007)
-
Proteolytic maturation of transforming growth factor-α
Perspectives in Drug Discovery and Design (1995)
-
Polycations induce microvascular leakage of macromolecules in hamster cheek pouch
Inflammation (1991)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.