Abstract
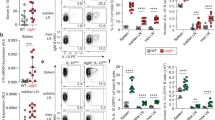
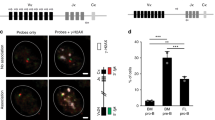
The μ and δ heavy chains of IgM and IgD, the first antibody isotypes expressed during bone-marrow B-cell development, are encoded by a common transcription unit. Expression of the μ chain on the surface of late pre-B cells allows their further development to immature B cells. Coexpression of the δ chain and emigration of the immature B cells to the periphery eventually leads to the development of naive mature IgM/IgD double-positive cells. Although IgM is important in driving B-cell development1, the contribution of IgD is not clear. Here we investigate the function of IgD. We generated mice deficient in IgM (IgM−/− mice) by deleting the μ region in embryonic stem cells. IgM−/− mice showed normal B-cell development and maturation, with IgD replacing membrane-bound and secretory IgM. Moreover, specific B-cell responses and isotype class switches occurred during immunization or infection. In contrast to mice deficient in B cells, IgM−/− mice survived infection with vesicular stomatitis virus by developing neutralizing immunoglobulins, but they were more susceptible than wild-type controls with delayed specific immunoglobulin responses. These data lead us to conclude that IgD is largely able to substitute for IgM functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Iglesias, A. et al. Early B cell development requires mu signalling. Eur. J. Immunol. 23, 2622–2630 (1993).
Ashfield, R. et al. MAZ-dependent termination between closely spaced human complement genes. EMBO J. 13, 5656–5667 (1994).
Yuan, D., Witte, P. L., Tan, J., Hawley, J. & Dang, T. Regulation of IgM and IgD heavy chain gene expression: effect of abrogation of intergenic transcriptional termination. J. Immunol. 157, 2073–2081 (1996).
Mansour, S. L., Thomas, K. R. & Capecchi, M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336, 348–352 (1988).
Sauer, B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 225, 890–900 (1993).
Gu, H., Zou, Y. R. & Rajewsky, K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73, 1155–1164 (1993).
Noben-Trauth, N., Kohler, G., Burki, K. & Ledermann, B. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Trans. Res. 5, 487–491 (1996).
8. Hayakawa, K. et al. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl Acad. Sci. USA 81, 2494–2498 (1984).
Hayakawa, K., Hardy, R. R. & Herzenberg, L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur. J. Immunol. 16, 450–456 (1986).
Ahearn, J. M. et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity 4, 251–262 (1996).
Engel, P. et al. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39–50 (1995).
Rickert, R. C., Rajewsky, K. & Roes, J. Impairment of T-cell-dependent B cell responses and B-1 cell development in CD19-deficient mice. Nature 376, 352–355 (1995).
Weiss, E. A., Tucker, P. W., Finkelman, F. D. & Yuan, D. Analysis of immunoglobulin heavy chain delta transcription termination in the production of delta S or delta M mRNA. Mol. Immunol. 28, 687–695 (1991).
Iglesias, A., Lamers, M. & Kohler, G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature 330, 482–484 (1987).
Randall, T. D., King, L. B. & Corley, R. B. The biological effects of IgM hexamer formation. Eur. J. Immunol. 20, 1971–1979 (1990).
Bachmann, M. F. et al. The role of antibody concentration and avidity in antiviral protection. Science 276, 2024–2027 (1997).
Freer, G. et al. Vesicular stomatitis virus Indiana glycoprotein as a T-cell-dependent and -independent antigen. J. Virol. 68, 3650–3655 (1994).
Leist, T. P., Cobbold, S. P., Waldmann, H., Aguet, M. & Zinkernagel, R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 138, 2278–2281 (1987).
Kalinke, U. et al. Monovalent single-chain Fv fragments and bivalent miniantibodies bound to vesicular stomatitis virus project against lethal infection. Eur. J. Immunol. 26, 2801–2806 (1996).
Brundler, M. A. et al. Immunity to viruses in B cell-deficient mice; influence of antibodies on virus persistance and on T cell memory. Eur. J. Immunol. 26, 2257–2262 (1996).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. AB cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, 423–426 (1991).
Nitschke, L., Kosco, M. H., Kohler, G. & Lamers, M. C. Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc. Natl Acad. Sci. USA 90, 1887–1891 (1993).
Roes, J. & Rajewsky, K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J. Exp. Med. 177, 45–55 (1993).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Reth, M. Antigen receptors on B lymphocytes. Annu. Rev. Immunol. 10, 97–121 (1992).
Achatz, G., Nitschke, L. & Lamers, M. C. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science 276, 409–411 (1997).
Mummery, C. L., Feyen, A., Freund, E. & Shen, S. Characteristics of embryonic stem cell differentiation: a comparison with two embryonal carcinoma cell lines. Cell Diff. Dev. 30, 195–206 (1990).
Nitschke, L., Kopf, M. & Lamers, M. C. Quick nested PCR screening of ES cell clones for gene targeting events. Biotechnology 14, 914–916 (1993).
Brombacher, F., Kohler, G. & Eibel, H. Bcell tolerance in mice transgenic for anti-CD8 immunoglobulin μ chain. J. Exp. Med. 174, 1335–1346 (1991).
Coico, R. F., Bhogal, B. S. & Thorbecke, G. J. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI. Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J. Immunol. 131, 2254–2257 (1983).
Acknowledgements
We thank M. Held, C. Westphal, I. Fidler, S. Meier, S. Herren and K.-H. Widmann for technical help, and L. Nitschke, P. Nielsen and M. Reth for discussion and critically reviewing the manuscript.
Author information
Authors and Affiliations
Author notes
This work is dedicated to the memory of Georges Khler who initiated this project.
- Frank Brombacher
Corresponding author
Rights and permissions
About this article
Cite this article
Lutz, C., Ledermann, B., Kosco-Vilbois, M. et al. IgD can largely substitute for loss of IgM function in B cells. Nature 393, 797–801 (1998). https://doi.org/10.1038/31716
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/31716
This article is cited by
-
The importance of B cell receptor isotypes and stereotypes in chronic lymphocytic leukemia
Leukemia (2019)
-
Beyond binding: antibody effector functions in infectious diseases
Nature Reviews Immunology (2018)
-
Variant surface glycoprotein density defines an immune evasion threshold for African trypanosomes undergoing antigenic variation
Nature Communications (2017)
-
IgD attenuates the IgM-induced anergy response in transitional and mature B cells
Nature Communications (2016)
-
Responsiveness of B cells is regulated by the hinge region of IgD
Nature Immunology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.