Abstract
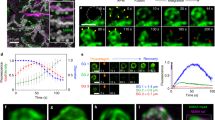
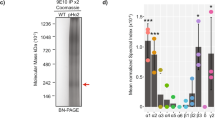
Spectrin is an ubiquitous protein composed of heterodimers with α and β subunits. It was first described in erythrocyte cell membranes (see ref. 1 for review) and subsequently in brain, intestinal brush borders, kidney, liver and adrenals2–8. Brain spectrin (fodrin) α-subunit, responsible for actin binding, has a relative molecular mass (Mr) of 240,000, whereas the β-subunit, involved in membrane attachment, has an Mr of 235,000 (refs 1, 3, 9–13). The membrane of secretory granules from adrenal chromaffin cells increases the viscosity of F-actin solution6,14, and spectrin-like protein is associated with storage granule and plasma membranes6. Here, we report the localization of fodrin in secretory cells using monospecific antibodies against the α-subunit of fodrin using indirect immunofluorescence. We find that the α-subunit forms an intensely stained continuous ring in the subplasmalemmal region of resting chromaffin cells. On stimulation of the cell with nicotine, high potassium or ionophores in the presence of calcium, fodrin forms patches. This aggregation is inhibited by trifluoperazine, hence the entrance of calcium into cells following cell depolarization seems to be the calmodulin-dependent stimulus initiating patch formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Branton, C., Cohen, C. M. & Tiler, J. Cell 24, 24–32 (1981).
Levine, J. & Willard, M. J. Cell Biol. 90, 631–643 (1981).
Burridge, K., Kelly, T. & Mangeat, P. J. Cell Biol. 95, 478–486 (1982).
Hirokawa, N., Cheney, R. E. & Willard, M. Cell 32, 953–965 (1983).
Lazarides, E. & Nelson, J. Science 220, 1295–1296 (1983).
Aunis, D. & Perrin, D. J. Neurochem. 42, 1558–1569 (1984).
Lazarides, E., Nelson, W. J. & Kasamatsu, T. Cell 36, 269–277 (1984).
Glenney, J. R., Glenney, P. & Weber, K. J. biol. Chem. 257, 9781–9787 (1982).
Glenney, J. R., Glenney, P. & Weber, K. J. Cell Biol. 96, 1491–1496 (1983).
Fowler, V. M., Luna, E. J., Hargreaves, W. R., Taylor, D. L. & Branton, D. J. Cell Biol. 88, 388–395 (1981).
Calvert, R., Bennett, P. & Gratzer, W. Eur. J. Biochem. 107, 355–361 (1980).
Litman, D., Hsu, C. J. & Marchesi, V. T. J. Cell Sci. 42, 1–22 (1980).
Morrow, J. S., Speicher, D. W., Knowles, W. J., Hsu, C. J. & Marchesi, V. T. Proc. natn. Acad. Sci. U.S.A. 77, 6592–6596 (1980).
Fowler, V. M. & Pollard, H. B. Nature 295, 336–339 (1982).
Livett, B. G. in Cell Biology of the Secretory Process (ed. Cantin, M.) 309–351 (Karger, Basel, 1984).
Winkler, H. & Carmichael, S. W. in The Secretory Granule (eds Poisner, A. & Trifaro, J. M.) 3–79 (Elsevier, Amsterdam, 1982).
Fenwick, C. M., Fajdiga, P. B., Howe, N. B. S. & Livett, B. G. J. Cell Biol. 76, 12–30 (1978).
Aunis, D., Guerold, B., Bader, M. F. & Ciesielski-Treska, J. Neuroscience 5, 2261–2277 (1980).
Kenigsberg, R. L. & Trifaro, J. M. Neuroscience 5, 1457–1566 (1980).
Unsicker, K., Griesser, G. H., Lindmar, R., Loffelholz, K. & Wolf, J. Neuroscience 5, 1445–1460 (1980).
Phillips, J. H., Burridge, K., Wilson, S. P. & Kirshner, N. J. Cell Biol. 97, 1906–1917 (1983).
Schneider, A. S., Cline, H. T., Rosenheck, K. & Sonenberg, M. J. Neurochem. 37, 567–575 (1981).
Knight, D. E. & Kesteven, N. T. Proc. R. Soc. B218, 177–199 (1983).
Carlin, R. K., Bartelt, D. C. & Siekevitz, P. J. Cell Biol. 96, 443–448 (1983).
Kenigsberg, R. L., Côté, A. & Trifaro, J. M. Neuroscience 7, 2277–2286 (1982).
Langley, O. K. & Aunis, D. Cell Tissue Res. 238, 497–502 (1984).
Levine, J. & Willard, M. Proc. natn. Acad. Sci. U.S.A. 80, 191–195 (1983).
Repasky, E. A., Symer, D. E. & Bankert, R. B. J. Cell Biol. 99, 350–355 (1984).
Burgoyne, R. D., Geisow, M. J. & Barron, J. Proc. R. Soc. B216, 111–115 (1982).
Bader, M. F. & Aunis, D. Neuroscience 8, 165–181 (1983).
Bader, M. F., Ciesielski-Treska, J., Thiersé, D., Hesketh, J. E. & Aunis, D. J. Neurochem. 37, 917–933 (1981).
Davis, J. & Bennett, V. J. biol. Chem. 258, 7757–7766 (1983).
Laemmli, U. K. Nature 227, 680–685 (1970).
Towbin, M., Staehelin, T. & Gordon, J. Proc. natn. Acad. Sci. U.S.A. 76, 4350–4354 (1979).
Fuller, G. H., Brinkley, B. R. & Boughter, J. M. Science 187, 948–951 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perrin, D., Aunis, D. Reorganization of α-fodrin induced by stimulation in secretory cells. Nature 315, 589–592 (1985). https://doi.org/10.1038/315589a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/315589a0
This article is cited by
-
The role of F-actin in the transport and secretion of chromaffin granules: an historic perspective
Pflügers Archiv - European Journal of Physiology (2018)
-
Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells
Experimental & Molecular Medicine (2013)
-
The F-Actin Cortex in Chromaffin Granule Dynamics and Fusion: a Minireview
Journal of Molecular Neuroscience (2012)
-
Autoantibodies to alfa-fodrin in patients with Hashimoto thyroiditis and Sjögren’s syndrome: possible markers for a common secretory disorder
Rheumatology International (2008)
-
Sjögren’s Syndrome—Study of Autoantigens and Autoantibodies
Clinical Reviews in Allergy & Immunology (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.