Abstract
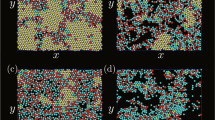
The properties of two-dimensional arrays of micrometre-sized particles are of interest in relation to a wide range of phenomena, including self-organization and phase behaviour in colloid science and condensed-matter physics1,2,3, the behaviour of dusty plasmas4 and the templating of ordered structures for photonic applications5. Most studies have used pre-existing particles such as monodisperse latex spheres, which may be manipulated with electric or magnetic fields. In contrast, we report here an inorganic solution that spontaneously precipitates a self-organized two-dimensional colloid crystal at the air/water interface. A solution of calcium hydroxide exposed to air reacts with dissolved carbon dioxide to precipitate microcrystals of calcium carbonate in the form of calcite. These aggregate at the surface to form a disordered gel mat with fractal characteristics6. We find, however, that in aged solutions a second population of charged microcrystals with the ‘dogtooth spar’ morphology appears on the surface. These crystallites, which can be observed by optical microscopy, become organized into a regular triangular lattice. The competition between electrostatic and capillary forces between particles leads to lattice spacings of the order of 125 to 175 µm, 5 to 7 times the diameter of the particles. These structures are stable for around 24 h, but eventually aggregate with the fractal gel. The mechanism of their self-organization, as yet incompletely understood, might provide some insights into similar phenomena in colloid science2,3,7,8,9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Skeltorp, A. T. One- and two-dimensional crystallization of magnetic holes. Phys. Rev. Lett. 51, 2306–2309 (1983).
Murray, C. A. & Van Winkle, D. H. Experimental observation of two-stage melting in a classical two-dimensional screened Coulomb system. Phys. Rev. Lett. 58, 1200–1203 (1986).
Murray, C. A. & Grier, D. G. Video microscopy of monodisperse colloidal systems. Annu. Rev. Phys. Chem. 47, 421–462 (1996).
Thomas, H. et al. Plasma crystal: Coulomb crystallization in a dusty plasma. Phys. Rev. Lett. 73, 652–655 (1994).
Van Blaaderen, A. & Wiltzius, P. Template-directed colloidal crystallization. Nature 385, 321–324 (1997).
Nakayama, T., Nakahara, A. & Matsushita, M. Cluster-cluster aggregation of calcium carbonate colloid particles at the air/water interface. J. Phys. Soc. Jpn 64, 1114–1119 (1996).
Larson, A. E. & Grier, D. G. Like-charge attractions in metastable colloidal crystallites. Nature 385, 230–233 (1997).
Kepler, G. M. & Fraden, S. Attractive potential between confined colloids at low ionic strength. Phys. Rev. Lett. 73, 356–359 (1994).
Murray, C. A. When like charges attract. Nature 385, 203–204 (1997).
Moody, J. R. & Lindstrom, R. M. Selection and cleaning of plastic containers for storage of trace element samples. Anal. Chem. 77, 2264–2267 (1977).
Sleytr, U. B. & Messner, P. Crystalline bacterial cell surface layers (s-layers). Ency. Microbiol. 1, 605–613 (1992).
Medina-Noyola, M. & Ivlev, B. I. Interaction in colloidal systems: bucking and melting. Phys. Rev. E 52, 6281–6288 (1995).
Camion, C., Roussel, J. F., Faure, R. & Blanc, R. Mesure des forces d'attraction entre spheres partiellement immergees: Influence des interfaces. Europhys. Lett. 3, 449–457 (1987).
Chan, D. Y. C., Henry, J. D. J & Whie, J. R. The interaction of colloidal particles collected at fluid interfaces. J. Colloid Interface Sci. 79, 410–418 (1981).
Israelachvili, J. N. Intermolecular and Surface Forces (2nd edn) (Academic, London, 1992).
Acknowledgements
The hospitality of the Naval Research Laboratory and partial support from the Office of Naval Research during a sabbatical visit of H.H.W. are gratefully acknowledged. We thank K.Hathaway, B. Gaber, P. Schoen, D. Turner and G. Lee for useful discussions and access to instruments used in the study. J.N.K. was a summer student whose visit to the Naval Research Laboratory was supported by an Undergraduate Minority Student Program Grant from the Chemical and Transport Systems Division, National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wickman, H., Korley, J. Colloid crystal self-organization and dynamics at the air/water interface. Nature 393, 445–447 (1998). https://doi.org/10.1038/30930
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/30930
This article is cited by
-
Single-particle tracking of the formation of a pseudoequilibrium state prior to charged microgel cluster formation at interfaces
NPG Asia Materials (2020)
-
A physical biomarker of the quality of cultured corneal endothelial cells and of the long-term prognosis of corneal restoration in patients
Nature Biomedical Engineering (2019)
-
Complex electric double layers in charged topological colloids
Scientific Reports (2018)
-
Dynamic control of the photonic smectic order of membranes
Nature Materials (2005)
-
Electric-field-induced capillary attraction between like-charged particles at liquid interfaces
Nature (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.