Abstract
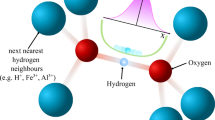
Cyclodextrins have proved useful as model systems for the study of hydrogen bonding1, α-Cyclodextrin (α-CD) crystal-lizes from water as a hexahydrate having well defined hydrogen bonds (O—H…O) in linear and circular arrangements favouring ‘endless’ …O—H…O—H…O—H… chains1–3. The larger β-cyclodextrin (β-CD) forms a crystalline dodecahydrate, β-CD·12H2O in which X-ray localization of the hydroxyl hydrogen atoms is made difficult because 25 of the 45 hydroxyl groups are statistically disordered and water molecules enclosed within the β-CD cavity are distributed over several sites that are not fully occupied4. We have therefore carried out a neutron diffraction study of β-CD at the Oak Ridge high flux isotope reactor to determine whether there is any well defined hydrogen bonding in disordered systems. In β-CD there are 19 hydrogen bonds of the type O—H…H—O. In these bonds, oxygen atoms are in the normal O—H…O distance range5, but two statistically half-occupied H atoms are arranged between them. The fact that the H…H separation of ∼1 Å is so short that the two H atoms positions are mutually exclusive suggests an equilibrium between two states: O—H…O⇌O…H—O. Of the two H atoms only one is in hydrogen-bonding contact at a given time; the other one is flipped out to form a hydrogen bond with an adjacent acceptor group and vice versa. Because long hydrogen-bonding chains are involved in a cooperative, concerted motion (domino effect), we have coined the term ‘flip-flop hydrogen bond’. This study demonstrates that hydrogen bonds are opera-tive in disordered systems and display dynamics even in solid state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Klar, B., Hingerty, B. & Saenger, W. Acta crystallogr. B36, 1154–1165 (1980).
Saenger, W. Nature 279, 343–344 (1979).
Lesyng, B. & Saenger, W. Biochim. biophys. Acta (in the press).
Lindner, K. & Saenger, W. Carbohydrate Res. (in the press).
Jeffrey, G. A. & Tagaki, S. Acc. chem. Res. 11, 264–270 (1978).
Weast, R. C. (ed.) CRC Handbook of Chemistry and Physics, D-178 (CRC Press, Cleveland, 1976).
Peterson, S. W. & Levy, H. A. Acta crystallogr. 10, 70–76 (1957).
Kuhs, W. F. & Lehmann, M. L. Nature 294, 432–436 (1981).
Hollander, F. & Jeffrey, G. A. J. chem. Phys. 66, 4699–4705 (1977).
Pauling, L. J. Am. Chem. Soc. 57, 2680–2684 (1935); The Nature of Chemical Bond 3rd edn, 467 (Cornell University Press, 1960).
Yamazaki, M. et al. Chem. Lett, (in the press).
Chacko, K. K. & Saenger, W. J. Am. chem. Soc. 103, 1708–1715 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saenger, W., Betzel, C., Hingerty, B. et al. Flip-flop hydrogen bonding in a partially disordered system. Nature 296, 581–583 (1982). https://doi.org/10.1038/296581a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/296581a0
This article is cited by
-
Alternative hydrogen bond models of cellulose II and IIII based on molecular force-fields and density functional theory
Cellulose (2015)
-
Coordinated proton tunnelling in a cyclic network of four hydrogen bonds in the solid state
Nature (1999)
-
Crystal and molecular structures of three modifications of the androgen dehydroepiandrosterone (DHEA)
Journal of Chemical Crystallography (1995)
-
Effects of N substituents on structure in 2-N-substituted 8-oxyquinolines: Conjugation between the unshared electron pair on the nitrogen atom and the aromatic ring
Journal of Structural Chemistry (1989)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.