Abstract
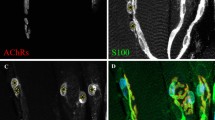
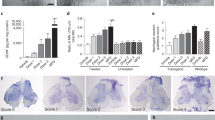
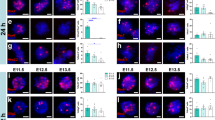
The twitcher mouse is a mutant affected by a form of leukodystrophy which shows close similarities to human globoid cell (Krabbe&'s) leukodystrophy. Transmission is by an autosomal recessive gene twi. Progressive loss of myelin sheaths from both central and peripheral nervous systems and the presence of inclusion-laden macrophages are characteristic findings. Morphological features of the twitcher have been described by Duchen et al.1. Nerve iso- and allografting have been used to determine the roles of axon and Schwann cell in a number of mouse2 and human3 nerve abnormalities. Schwann cells in a graft proliferate and become associated with regenerating host axons which grow through the graft into the host distal stump. In the twitcher, peripheral nerve axons do not degenerate but are thinner than normal, although there is considerable axonal degeneration in the central nervous system. In 15-day-old mutants, inclusions have been found in Schwann cells associated with apparently normal myelin sheaths. Grafting experiments might show whether the phenotype of this mutant is fully expressed in the Schwann cell, or if axons are also involved. In previous experiments, survival of transplanted Schwann cells was achieved by the use of T cell-suppressed2,3 or nude mice4. We report here that a twitcher nerve transplanted in immunologically unsuppressed animals reproduces all the characteristic features of leukodystrophy and conversely that Schwann cells from unaffected mice can produce normal myelin when associated with twitcher axons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Duchen, L. W., Eicher, E. M., Jacobs, J. M., Scaravilli, F. & Teixeira, F. Brain 103, 695–710 (1980).
Aguayo, A. J., Attwell, M., Trecarten, J., Perkins, S. & Bray, G. M. Nature 265, 73–75 (1977).
Aguayo, A. J., Kasarjian, J., Skamene, E., Kongshavn, P. & Bray, G. M. Nature 268, 753–755 (1977).
Dyck, P. K., Lais, A. C. & Low, P. A. Neurology 28, 261–265 (1978).
Schochet, S. S., McCormick, W. F. & Powell, G. F. Acta neuropath. 36, 153–160 (1976).
Yajima, K., Fletcher, T. F. & Suzuki, K. J. neurol. Sci. 33, 179–197 (1977).
Suzuki, K. & Suzuki, Y. Proc. natn. Acad. Sci. U.S.A. 66, 302–309 (1970).
Malone, M. Trans. Am. Soc. Neurochem. 1, 56 (1970).
Kobayashi, T., Scaravilli, F. & Suzuki, K. Proc. 1st Symp. Neurological Mutations Affecting Myelination, Seillac (Abstr.) 257–262 (Elsevier/North-Holland, Amsterdam, 1980).
Romine, J. S., Aguayo, A. J. & Bray, G. M. Brain Res. 98, 601–606 (1975).
Grooth, C.-G. et al. Lancet i, 1260–1264 (1971).
Grooth, C.-G. et al. Transplantn Proc. 11, 1218–1219 (1979).
Guttman, R. D. Transplant Proc. 11, 1622–1625 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scaravilli, F., Jacobs, J. Peripheral nerve grafts in hereditary leukodystrophic mutant mice (twitcher). Nature 290, 56–58 (1981). https://doi.org/10.1038/290056a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/290056a0
This article is cited by
-
Central Nervous System-directed AAV2/5-Mediated Gene Therapy Synergizes with Bone Marrow Transplantation in the Murine Model of Globoid-cell Leukodystrophy
Molecular Therapy (2007)
-
Disorders in myelination in thetwitcher mutant: Immunohistochemical and biochemical studies
Neurochemical Research (1985)
-
Defective Schwann cell function in canine inheritedHypertrophic Neuropathy
Acta Neuropathologica (1984)
-
Biochemical aspects of globoid and metachromatic leukodystrophies
Neurochemical Pathology (1984)
-
Enzyme replacement in grafted nerve of twitcher mouse
Nature (1983)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.