Abstract
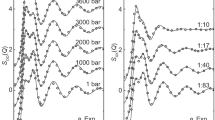
In spite of much experimental and theoretical work during the past 80 yr, there is at present no generally agreed structural model for concentrated aqueous solutions, even for a relatively simple system like NaCl dissolved in H2O (ref. 1). Aqueous solutions contain four chemical species and so require 10 pair correlation functions to describe the structure, thus complicating attempts to isolate, for example, ion–water correlations unambiguously from diffraction experiments. The combination of long-range Coulomb potentials, quantum-mechanical hydrogen bonding effects and the intractability of the liquid state has proved an obstacle to theoretical understanding. We have investigated the coordination of water molecules around Cl– ions in aqueous solution by the isotopic substitution method. A range of counter-ions and concentrations was used to answer questions about the sensitivity of Cl– hydration to the nature of the counter-ion and the ionic strength. We show here that Cl– hydration is essentially independent of the cation and of ionic strength with the possible exception of a transition metal counter ion (Ni2+). The insensitivity of Cl– hydration to cation type should lead to a major simplification in the theory of solutions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Franks, F.(ed.)Water A Comprehensive Treatise Vols 1–6 (Plenum, New York, 1972–79).
Narten, A. H., Vaslow, F. & Levy, H. A. J. chem. Phys. 58, 5017 (1973).
Enderby, J. E., Howells, W. S. & Howe, R. A. Chem. Phys. Lett. 21, 109 (1973).
Soper, A. K., Neilson, G. W., Enderby, J. E. & Howe, R. A. J. phys. C Solid State Phys. 10, 1973 (1977).
Neilson, G. W. & Enderby, J. E. J. Phys. C Solid State Phys. 11, L625 (1978).
Bopp, P., Heinzinger, K. & Jancsò, G. Z. Naturf. 32 A, 620 (1977).
Pálinkás, G., Radnai, T. & Hajdu, F. Z. Naturf. 35 A, 107 (1980).
Cummings, S., Enderby, J. E. & Howe, R. A. J. Phys. C Solid State Phys. 13, 1 (1980).
Enderby, J. E. & Neilson, G. W. Water, A Comprehensive Treatise Vol. 6 (ed. Franks, F.) (Plenum, New York, 1979).
Kistenmacher, H., Popkie, H. & Clementi, E. J. chem. Phys. 61, 799 (1974).
Bopp, B., Dietz, W. & Heinzinger, K. Z. Naturf. 34 b, 1424 (1979).
Neilson, G. W. Chem. Phys. Lett. 68, 247 (1979).
Hertz, H. G. Water, A Comprehensive Treatise Vol. 3 (ed.Franks, F.) (Plenum, New York, 1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cummings, S., Enderby, J., Neilson, G. et al. Chloride ions in aqueous solutions. Nature 287, 714–716 (1980). https://doi.org/10.1038/287714a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/287714a0
This article is cited by
-
Dissolving salt is not equivalent to applying a pressure on water
Nature Communications (2022)
-
Non-metallic charge carriers for aqueous batteries
Nature Reviews Materials (2020)
-
Cooperative interaction of sodium and chlorine ions with β-cellobiose in aqueous solution from quantum mechanics and molecular dynamics
Cellulose (2020)
-
Volume, electrostriction, and solvation relations along the liquid-vapor critical curve of aqueous sodium chloride solutions
Journal of Solution Chemistry (1993)
-
Neutron diffraction methods applied to aqueous solutions
International Journal of Thermophysics (1988)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.