Abstract
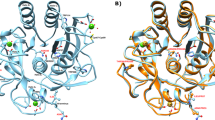
MOST proteins are denatured at temperatures above 50–60°C, although some enzymes, especially those from thermophilic organisms, remain active at temperatures up to 80–90 °C. The determination of the three-dimensional structure of the thermostable protease thermolysin showed that heat-stable proteins do not contain unusual structural features absent from less stable proteins1,2. Furthermore, the amino acid sequences of similar proteins from both mesophilic and thermophilic sources have been shown to be homologous, suggesting that the respective structures are similar3,4. Nevertheless, such homologous amino acid sequences also include many differences which obscure those amino acid changes actually responsible for differences in thermostability. We report here the structure of a temperature sensitive (ts) mutant of T4 phage lysozyme. This permits the first direct comparison of two protein structures in which all differences are directly related to a change in thermal stability. It is shown that, except for the replacement of a partially exposed arginine by a histidine, the three-dimensional structure of the ts lysozyme is virtually identical with that of native lysozyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Matthews, B. M., Jansonius, J. N., Colman, P. M., Schoenborn, B. P. & Dupourque, D. Nature new Biol. 238, 37–41 (1972).
Matthews, B. W., Weaver, L. H. & Kester, W. R. J. biol. Chem. 249, 8030–8044 (1974).
Tanaka, M. et al. J. biol. Chem. 246, 3953–3960 (1971).
Bridgen, J. & Harris, J. I. Abs. Ninth Int. Congr. Biochem., Stockholm, p. 59 (1973).
Koch, G. & Dreyer, W. J. Virology 6, 291–293 (1958).
Tsugita, A. & Inouye, M. J. molec. Biol. 37, 201–212 (1968).
Matthews, B. W. & Remington, S. J. Proc. natn. Acad. Sci. U.S.A. 71, 4178–4182 (1974).
Remington, S. J. et al. J. molec. Biol. 118, 81–98 (1978).
Elwell, M. & Schellman, J. Biochim. biophys. Acta 386, 309–323 (1975).
Streisinger, G., Mukai, F., Dreyer, W. J., Miller, B. & Horiuchi, S. Cold Spring Harb. Symp. quant. Biol. 26, 25–30 (1961).
Tsugita, A. Enzymes 5, 3rd edn, 343–411 (1971).
Tsugita, A. & Inouye, M. J. biol. Chem. 243, 391–397 (1968).
Matthews, B. W. in The Proteins Vol. 3 (eds Neurath, H. & Hill, R. L.) 3rd edn, 403–590 (Academic, New York, 1977).
Weaver, L. H., Kester, W. R., Ten Eyck, L. F. & Matthews, B. W. Experientia suppl. 26, 31–39 (1976).
Perutz, M. F. & Raidt, H. Nature 255, 256–259 (1975).
Schellman, J. A. Compt. rend. Trav. Lab. Carlsberg Ser. Chim. 29, 230–259 (1955).
Kauzmann, W. Adv. Protein Chem. 14, 1–63 (1959).
Brandts, J. F. in Thermobiology (ed. Rose, A. H.) 25–72 (Academic, New York, 1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GRÜTTER, M., HAWKES, R. & MATTHEWS, B. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature 277, 667–669 (1979). https://doi.org/10.1038/277667a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/277667a0
This article is cited by
-
Intrinsic basis of thermostability of prolyl oligopeptidase from Pyrococcus furiosus
Scientific Reports (2021)
-
Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran
BMC Chemistry (2020)
-
Biochemical characterization of thermostable β-1,4-mannanase belonging to the glycoside hydrolase family 134 from Aspergillus oryzae
Applied Microbiology and Biotechnology (2017)
-
Directed evolution studies with combinatorial libraries of T4 lysozyme mutants
Molecular Diversity (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.