Abstract
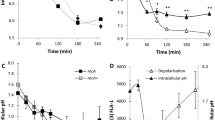
STUDIES with microbial mutants1–3 and animal cells4,5 have shown that spermidine and spermine , and perhaps their biosynthetic precursor putrescine, are required for optimal cell proliferation. Mutants of Escherichia coli blocked in the synthesis of putrescine provide an important tool with which to study the cellular function of these compounds. Previous work suggested a defect in DNA replication during starvation for polyamines; infection of spermidine-starved E. coli with bacteriophage T4 resulted in a submaximal rate of DNA accumulation6, while polyamine-limited growth of E. coli resulted in about twice as much DNA per cell as expected from the growth rate2. Also, increased cellular DNA has been seen during thymine-limited chromosome replication7 and in strains possessing mutations in the rep locus8. In both cases, this change in cellular composition seemed to be due to a reduced rate of replication fork movement and an increased number of forks per cell9,10. We show here that the abnormally high DNA content during polyamine limitation also seems to be the result of decreased replication fork velocity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Maas, W. K., Leifer, Z. & Poindexter, J. Ann. N.Y. Acad. Sci. 171, 957–967 (1970).
Morris, D. R. & Jorstad, C. J. Bact. 113, 271–277 (1973).
Whitney, P. & Morris, D. R. J. Bact. (in the press).
Fillingame, R. H., Jorstad, C. M. & Morris, D. R. Proc. natn. Acad. Sci. U.S A 72, 4042–4045 (1975).
Mamont, P. S. et al. Proc. natn. Acad. Sci. U.S.A. 73, 1626–2630 (1976).
Dion, A. S. & Cohen, S. S. J. Virol. 9, 423–427 (1972).
Zaritsky, A. & Pritchard, R. H. J. Bact. 114, 824–837 (1973).
Lane, H. E. D. & Denhardt, D. T. J. Bact. 120, 805–814 (1974).
Pritchard, R. H. & Zaritzky, A. Nature 226, 126–131 (1970).
Lane, H. E. D. & Denhardt, D. T. J. molec. Biol. 97, 99–112 (1975).
Burton, K. Biochem. J. 62, 315–323 (1956).
Cooper, S. & Helmstetter, C. E. J. molec. Biol. 31, 519–540 (1968).
Lark, K. G. & Bird, R. E. Proc. natn. Acad. Sci. U.S.A. 54, 1444–1450 (1965).
Neidhardt, F. C., Block, P. L. & Smith, D. F. J. Bact. 119, 736–747 (1974).
Maas, W. K. Molec. Gen. Genet. 119, 1–9 (1972).
Caro, L. G. & van Tubergen, R. P. J. Cell Biol. 15, 173–188 (1962).
Caro, L. G. J. molec. Biol. 48, 329–338 (1970).
Sueoka, N. & Yoshikawa, H. Genetics 52, 747–757 (1965).
Scheckman, R. et al. Proc. natn. Acad. Sci. U.S.A. 69, 2691–2695 (1972).
Schekman, R., Weiner, A. & Kornberg, A. Science 186, 987–993 (1974).
Geider, K. & Kornberg, A. J. biol. Chem. 249, 3999–4005 (1974).
Weiner, J. H., Bertsch, L. & Kornberg, A. J. blol. Chem. 250, 1972–1980 (1975).
Christiansen, C. & Baldwin, R. L. J. molec. Biol. 115, 441–454 (1977).
Gellert, M., Mizuuchi, K., O'Dea, M. & Nash, H. A. Proc. natn. Acad. Sci U.S.A. 73, 3872–3876 (1976).
Gellert, M., O'Dea, M., Itoh, T. & Tomizawa, J. Proc. natn. Acad. Sci. U.S.A. 73, 4474–4478 (1976).
Morris, D. R. & Hansen, M. T. J. Bact. 116, 588–592 (1973).
Krokan, H. & Eriksen, A. Eur. J. Biochem. 72, 501–508 (1977).
Knutson, J. C. & Morris, D. R. Biochim. biophys. Acta (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GEIGER, L., MORRIS, D. Polyamine deficiency reduces the rate of DNA replication fork movement in Escherichia coli. Nature 272, 730–732 (1978). https://doi.org/10.1038/272730a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/272730a0
This article is cited by
-
The increase in putrescine content in the gastric mucosa of rats with ulcerations induced by restraint-immersion stress
Gastroenterologia Japonica (1981)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.