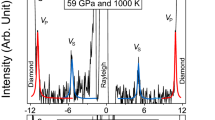
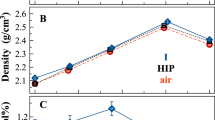
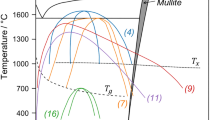
Abstract
THERE are many studies1–7 of the presence of inert gases in glasses. Because they have no electron affinity and high ionisation potentials (12 eV for Xe to 24 eV for He) the solubility of inert gases in glasses has been considered a simple physical process with no strong chemical interaction within the glass network. Recent work on pressure dependence of inert gas in vitreous1 and crystalline2 silica (SiO2) showed that the equilibrium solubility may be explained qualitatively by a statistical thermodynamic model3. At non-equilibrium conditions, however, a large quantity of inert gases can be incorporated into SiO2 by processes such as glow discharge8,9 and sputtering10,11, and here, the amount of inert gases in SiO2 far exceeds the equilibrium solubility. Using a high-rate sputtering technique, we prepared a SiO2 deposit containing 16.1 at. % Kr, expressed by a glass formula, Kr·2SiO2 and we report here the unique characteristics of this material.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shelby, J. E. J. appl. Phys. 47, 135 (1976).
Barrer, R. M. & Vaughan, D. E. W. Trans. Faraday Soc. 63, 2275 (1967).
Shackelford, F. J., Studt, R. L. & Fulrath, R. M. J. appl. Phys. 43, 1619 (1972).
Doremus, R. H. in Glass Science, ch. 8 (Wiley, New York, 1973).
Shelby, J. E. J. Am. ceram. Soc. 55, 61 (1972).
Shelby, J. E. Phys. Chem. Glasses 13, 167 (1972).
Swets, D. E., Lee, R. W. & Frank, R. C. J. chem. Phys. 34, 17 (1961).
Grant, W. A., Carter, G. Vacuum 15, 477 (1965).
Young, J. R. J. appl. Phys. 27, 926 (1956).
Hoffmeister, W. & Zuegel, M. Thin Solid Films 3, 35 (1969).
Petersson, S., Linker, G. & Meyer, O. Phys. Stat. Sol. a 14, 605 (1972).
McClanahan, E. D., Busch, R., Fairbanks, J. & Patten, J. W., presented at Gas Turbine Conference and Products Show, Zurich, Switzerland, March 3–April 14, 1974.
Wang, R. & Bayne, M. A. Proceedings of Second American Nuclear Society Meeting on Fusion Technology, p. 447 (1976).
Donohue, J. The Structure of Elements ch. 2, 27 (John Wiley, New York, 1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WANG, R. A vitreous Kr·2SiO2. Nature 270, 705–706 (1977). https://doi.org/10.1038/270705a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/270705a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.