Abstract
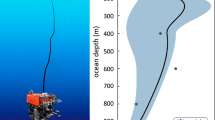
PARTICULATE matter in the waters of the Gulf of St Lawrence, a major marginal sea, contains as much as 16,500 p.p.m. manganese. This is comparable with the levels of manganese found in deep-sea sediments1. Here we show that it is possibly a related phenomenon. Suspended particulate matter was collected by filtration on 0.4-µm Nucleopore filters on a series of six stations along the axis of the Laurentian Trough. The samples were digested in a Teflon bomb with a mixture of aqua regia and hydrofluoric acid, and analysed by atomic absorption spectrophotometry2. Figure 1 shows a typical vertical profile of the concentration of suspended particulate matter and its content of manganese. The manganese content increases rapidly with depth to a maximum value of 16,500 p.p.m. at 40–120 m above the bottom. The profiles are similar for the two cruises.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Goldberg, E. D. & Arrhenius, G. Geochim. cosmochim. Acta 13, 153–212 (1958).
Rantala, R. R. & Loring, D. H. At. Absorp. Newslett. 16, 51–52 (1977).
Sundby, B. Can. J. Earth Sci. 11, 1517–1533 (1974).
Feely, R. A. Mar. Chem. 3, 121–156 (1975).
Evans, D. W., Cutshall, N. H., Cross, F. A. & Wolfe, D. A., Estuar. Coast. Mar. Sci. 5, 71–80 (1977).
Graham, W. F., Bender, M. L. & Klinkhammer, G. P. Limnol. Oceanogr. 21, 665–673 (1976).
Bewers, J. M. & Yeats, P. A. Nature 268, 595–598 (1977).
Chesselet, R., Spencer, D. W. & Biscaye, P. E. Special Symp. Geochemistry and Ocean Mixing. Joint Oceanographic Assembly (Edinburgh, 1976).
Turekian, K. K. in Progress in Oceanography (ed. Sears, M.) 227–244, (Pergamon, Oxford, 1967).
Goldberg, E. D. J. Geol. 62, 249–265 (1954).
Turekian, K. K. Geochim. cosmochim. Acta 41, 1139–1144 (1977).
Jenne, E. A. Am. Chem. Soc., Adv. Chem. Ser. 73, 337–387 (1968).
Elderfield, H. Mar. Chem. 4, 103–132 (1976).
Broecker, W. S. Chemical Oceanography 97–103 (Harcourt, Brace Jovanovich, New York, 1974).
Goldberg, E. D. Comments Earth Sci. Geophys. 1, 117 (1971).
Aston, S. R. & Chester, R. in Estuarine Chemistry (eds Burton, J. D. & Liss, P. S.) 37–52 (Academic, London, 1976).
Rantala, R. R. & Loring, D. H. At. Absorp. Newslett. 14, 117–119 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SUNDBY, B. Manganese-rich particulate matter in a coastal marine environment. Nature 270, 417–419 (1977). https://doi.org/10.1038/270417a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/270417a0
This article is cited by
-
The Speciation and Mobility of Mn and Fe in Estuarine Sediments
Aquatic Geochemistry (2019)
-
Preface to Bjørn Sundby’s Special Issue of Aquatic Geochemistry
Aquatic Geochemistry (2012)
-
Redox potential measurement in natural waters
Fresenius' Journal of Analytical Chemistry (1991)
-
Seasonal cycle of manganese in seawater in Harima Sound
Journal of the Oceanographical Society of Japan (1982)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.