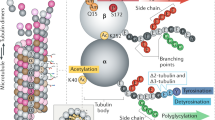
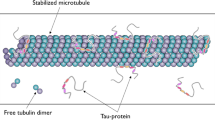
Abstract
THE finding by Borisy and Taylor1 that nervous tissue was the source of large amounts of soluble tubulin, the subunit protein of microtubules, has greatly facilitated the biochemical study of microtubular structure. From 10% to 20% of the total protein of developing brain is in the form of soluble tubulin2,3 and most studies have focused on this form of the protein. In 1970, however, Feit and Barondes demonstrated a large amount of colchicine binding activity, a term which has become associated with the presence of tubulin, in particulate fractions of brain4—fractions expected to be free of microtubule-derived tubulin. Recent studies indicate that polypeptides with the same size and drug-binding properties as soluble tubulin are present in synaptosomal plasma membranes5–7. These studies suggest a membrane-associated form of the protein which is insoluble in non-chaotropic buffers. I here report experiments which provide additional evidence for membrane-associated tubulin-like proteins and also suggest that a portion of this polypeptide(s) may be exposed on the external surface of the cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Borisy, G. C. & Taylor, E. W. J. Cell Biol. 34, 325–333 (1967).
Bamburg, J. R., Shooter, E. M. & Wilson, L. Biochemistry 12, 1476–1482 (1973).
Fellous, A., Francon, J., Virion, A. & Nunez, J. FEBS Lett. 57, 5–8 (1975).
Feit, H. & Barondes, S. H. J. Neurochem. 17, 1355–1364 (1970).
Blitz, A. L. & Fine, R. E. Proc. natn. Acad. Sci. U.S.A. 71, 4472–4476 (1974).
Bhattacharyya, B. & Wolff, J. J. biol. Chem. 250, 7639–7646 (1975).
Bhattacharyya, B. & Wolff, J. Nature 264, 576–577 (1976).
Patterson, P. H., Reichardt, L. F. & Chun, L. Y. Cold Spring Harb. Symp. quant. Biol. 30, 389–397 (1976).
Johnson, M., Ross, D., Meyers, M., Rees, R., Bunge, R., Wakshull, E. & Burton, H. Nature 262, 308–310 (1976).
Wood, P. M. & Bunge, R. Nature 256, 662–664 (1975).
Fine, R. E. & Bray, D. Nature new Biol. 234, 115–118 (1971).
Laemmli, U. K. Nature 227, 680–685 (1970).
Bryan, J. Fedn Proc. 33, 152–157 (1974).
Berlin, R. D., Oliver, J. M., Ukena, T. E. & Yin, H. H. Nature 247, 45–46, (1974).
Levi-Montalcini, R., Revotella, P. & Callisano, P. Rec. Prog. Hormone Res. 30, 635–682 (1974).
Gruenstein, E., Rich, A. & Weihling, R. R. J. Cell Biol. 64, 223–234 (1975).
Willingham, M. C., Ostlund, R. E. & Pastan, I. Proc. natn. Acad. Sci. U.S.A. 71, 4144–4148 (1974).
Bonner, W. M. & Laskey, R. A. Eur. J. Biochem. 46, 83–88 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ESTRIDGE, M. Polypeptides similar to the α and β subunits of tubulin are exposed on the neuronal surface. Nature 268, 60–63 (1977). https://doi.org/10.1038/268060a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/268060a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.