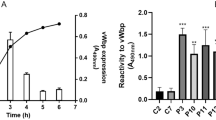
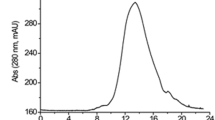
Abstract
THE fibrinogen molecule, which comprises 5% of plasma proteins, is involved in enzymatic reactions responsible for formation and dissolution of the fibrin clot1. Thereby, fibrinogen is being transformed by thrombin into fibrin, which is cross linked by glutaminyltransamidase (factor XIII), and ultimately degraded by plasmin. The Aα, Bβ and γ polypeptide chains (nomenclature according to the recommendations of the Committee on Nomenclature of the International Society on Thrombosis and Haemostasis, 1971) of fibrinogen involved in these reactions and the sites of enzymatic attack thereon are defined2–8. In addition to being involved in the enzymatic reactions responsible for formation and dissolution of the fibrin clot, fibrinogen exhibits the ability to interact in a non-enzymatic reaction with pathogenic staphylococci to cause their clumping9. Clumping of staphylococci is used to detect fibrinogen and its derivatives in blood and body fluids10,11. This reaction can detect as little as 0.03 µg human fibrinogen or its derivatives. The mechanism through which human fibrinogen interacts with staphylococci is however, unexplained and the localisation of the binding site(s) for staphylococci remains unknown. We therefore studied localisation of the binding site for staphylococci on human fibrinogen. We now report that the binding site for staphylococci is located on Aα and Bβ chains of human fibrinogen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blombäck, B., and Blombäck, M., Ann. N. Y. Acad. Sci., 202, 77–97 (1972).
Blombäck, B., Blombäck, M., Hessel, B., and Iwanaga, S., Nature, 215, 1445–1448 (1967).
Kudryk, B. J., Collen, D., Woods, K. R., and Blombäck, B., J. biol. Chem., 249, 3322–3325 (1974).
Doolittle, R. F., Chen, R., and Lau, F., Biochem. biophys. Res. Commun., 44, 94–100 (1971).
McKee, P. A., Mattock, P., and Hill, R. L., Proc. natn. Acad. Sci. U.S.A., 66, 738–744 (1971).
Mosesson, M. W., Finlayson, J. S., and Galanakis, D. K., J. biol. Chem., 248, 7913–7929 (1973)
Budzynski, A. Z., Marder, V. J., and Shainoff, J. R., J. biol. Chem., 249, 2294–2302 (1974).
Gaffney, P. J., Br. J. Haemat., 25, 277 (1973).
Duthie, E. S., J. gen. Microbiol., 13, 383–393 (1955).
Hawiger, J., Niewiarowski, S., and Thomas, D. P., J. Lab. clin. Med., 75, 93–108 (1970).
Thomas, D. P., Niewiarowski, S., Myers, A. R., Bloch, K. J., and Colman, R. W., New Engl. J. Med., 283, 663–668 (1970).
McFarlane, A. S., Nature, 182, 53 (1958).
Gollwitzer, R., Timpl, R., Becker, U., and Furthmayr, H., Eur. J. Biochem., 28, 497–506 (1972).
Lipinski, B., Hawiger, J., and Jeljaszewicz, J., J. exp. Med., 126, 979–998 (1967).
Allington, J. J., Br. J. Haemat., 13, 550–567 (1967).
Weber, K., and Osborn, M., J. biol. Chem., 244, 4406–4412 (1969).
Blombäck, B., Blombäck, M., Henschen, A., Hessel, B., Iwanaga, S., and Wood, K. R., Nature, 218, 130–134 (1968).
Barnhart, M. I., Sulisz, L., and Bluhm, G. B., in Immunopathology of Inflammation (edit. by Forscher, B. K., and Houck, J.), 59–65 (Excerpta Medica, Amsterdam, 1971).
Hawkey, C. M., in The Haemostatic Mechanism in Man and Other Animals, 217–229 (Academic Press, London and New York, 1970).
Hawiger, J., in Proc. Workshop on Animal Models of Thrombosis and Hemorrhagic Diseases (National Academy of Sciences, Washington DC, in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HAWIGER, J., HAMMOND, D. & TIMMONS, S. Human fibrinogen possesses binding site for staphylococci on Aα and Bβ polypeptide chains. Nature 258, 643–645 (1975). https://doi.org/10.1038/258643a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/258643a0
This article is cited by
-
Quantitative differences in specific binding of fibrinogen fragment D by M-positive and M-negative group-A streptococci
Medical Microbiology and Immunology (1984)
-
Binding of human fibrinogen and its polypeptide chains to group B streptococci
Medical Microbiology and Immunology (1983)
-
Interaction of an alkali stable polysaccharide from cell surface ofStaphylococci with human fibrinogen
Experientia (1978)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.