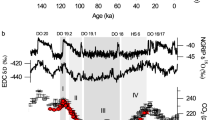
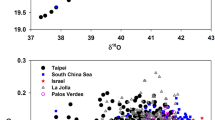
Abstract
THE concentration of CO2 in the atmosphere has increased from about 290 parts per million (p.p.m.) by volume in 1900 (ref. 1) to 323 p.p.m. in 1972 (ref. 2) and this increase is generally held to be due chiefly to combustion of fossil fuels. Indeed by 1970 this artificial mechanism had introduced to the atmosphere an amount of CO2 equivalent to 22% of the natural or pre-industrial atmospheric inventory3. About half of the added CO2 seems to have been transferred to the other major reservoirs of the dynamic carbon cycle, the oceans and biosphere. Before examining the threat to global climate and marine life4, future CO2 levels from this source must be predicted. Before such extrapolations can be confidently applied, however, we must fully understand the past response of the atmosphere to fossil CO2 emission. Since direct measurements of atmospheric CO2 are either not available or considered unreliable indirect methods of assessing the CO2 buildup must be invoked. Here a novel and potentially valuable approach is presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Callendar, G. S., Tellus, 10, 243 (1958).
Machta, L., in Carbon and the Biosphere, Brookhaven Symp. Biol., No. 24 (edit. by Woodwell, G. M., and Pecan, E. V.) (USAEC. Washington, DC., in the press).
Baxter, M. S., and Walton, A., Proc. R. Soc., A318, 213 (1970).
Broecker, W. S., Li, Y. H., and Peng, T. H., in Impingement of Man on the Oceans (edit. by Hood, D. W.), 287 (Wiley, New York, 1971).
Craig, H., Geochim. cosmochim. Acta, 3, 53 (1953).
Baxter, M. S., and Walton, A., Proc. R. Soc., A321, 105 (1971).
Craig, H., Geochim. cosmochim. Acta, 12, 133 (1957).
Dequasie, H. L., and Grey, D. C., Int. Lab., 20 (September/October 1971).
Keeling, C. D., Proc. Am. phil. Soc., 114, 10 (1970).
Peaceful Uses of Atomic Energy, 1, 3 (United Nations, 1956).
Revelle, R., in Restoring the Quality of Our Environment (President's Science Advisory Committee), 111 (White House, Washington, 1965).
Mitchell, J. M., jun., A. N.Y. Acad. Sci., 95, 235 (1961).
Craig, H., Science, N.Y., 119, 141 (1954).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FARMER, J., BAXTER, M. Atmospheric carbon dioxide levels as indicated by the stable isotope record in wood. Nature 247, 273–275 (1974). https://doi.org/10.1038/247273a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/247273a0
This article is cited by
-
Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China
Journal of Soils and Sediments (2010)
-
Combining sap flow measurement-based canopy stomatal conductance and 13C discrimination to estimate forest carbon assimilation
Science Bulletin (2005)
-
Climatic implications of δ13C and δ18O ratios from C3 and C4 plants growing in a tropical montane habitat in southern India
Journal of Biosciences (1999)
-
Purity assessments of major vegetable oils based on δ13C values of individual fatty acids
Journal of the American Oil Chemists' Society (1998)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.