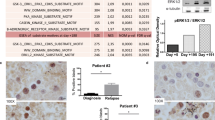
Abstract
Acute myeloid leukemia (AML) cells carry molecular defects that promote their leukemic proliferation, resistance to apoptosis and defect in differentiation. Pharmacological targeting of the nuclear factor kappaB (NF-κB) pathway has been shown to promote apoptosis of primary AML cells and to sensitize blasts to neoplastic drugs (Frelin, Blood 2005, 105, 804). The Fms-like tyrosine kinase 3 (FLT3), which sustains proliferation of normal hematopoietic progenitors is frequently overexpressed or mutated in AML patients. Using Ba/F3 murine pre-B cells transfected with various mutants of FLT3 (ITD, D835V, D835Y) and the MV4-11 human AML line, we show that normal or oncogenic stimulation of FLT3 led to activation of NF-κB. Pharmacological inhibition of either FLT3 with AG1296 or NF-κB with the small molecule inhibitor of IkappaB kinase-2 AS602868 reduced viability and triggered cell death. Moreover, AS602868 was also found to interfere directly with FLT3 kinase activation. AS602868 thus appears to target two different kinases that play a crucial role in the pathogenesis of AML, making it particularly attractive as a new therapeutical approach for AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Karin M . Nuclear factor-κB in cancer development and progression. Nature 2006; 441: 431–436.
Guzman M, Neering S, Upchurch D, Grimes B, Howard D, Rizzieri D et al. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001; 98: 2301–2307.
Frelin C, Imbert V, Griessinger M, Peyron A, Rochet N, Philip P et al. Targeting NF-κB activation via pharmacological inhibition of IKK2, induced apoptosis of human acute myeloid leukemia cells. Blood 2005; 105: 804–811.
Rayet B, Gélinas C . Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999; 18: 6938–6947.
Rosnet O, Buhring H, Marchetto S, Rappold I, Lavagna C, Sainty D et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia 1996; 10: 238–248.
Stirewalt D, Radich J . The role of Flt3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Tse K, Mukherjee G, Small D . Constitutive activation of Flt3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia 2000; 14: 1766–1776.
Gilliland D, Griffin J . Role of FLT3 in leukemia. Curr Opin Hematol 2002; 9: 274–281.
Levis M, Small D . FLT3 : ITDoes matter in leukemia. Leukemia 2003; 17: 1738–1752.
Levis M, Small D . FLT3 tyrosine kinase inhibitors. Int J Hematol 2005; 82: 100–107.
Tallman M, Gilliland D, Rowe J . Drug therapy for acute myeloid leukemia. Blood 2005; 106: 1154–1163.
Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner BM, Mueller-Dieckmann C et al. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell 1996; 86: 787–798.
Deininger M, Buchdunger E, Drucker B . The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005; 105: 2640–2653.
Sawyers C . Calculated resistance in cancer. Nat Med 2005; 11: 824–825.
Kim H, Hawke N, Baldwin A . NF-κB and IKK as therapeuti targets in cancer. Cell Death Differentiation 2006; 13: 738–747.
Wang CY, Mayo MW, Baldwin Jr AS . TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 1996; 274: 784–787.
Yamamoto Y, Gaynor R . Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest 2001; 107: 135–142.
Frelin C, Imbert V, Griessinger M, Loubat A, Dreano M, Peyron J-F . AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-alpha in Jurkat leukemic T cells. Oncogene 2003; 22: 8187–8194.
Takahashi S, Harigae H, Ishii K, Inomata M, Fujiwara T, Yokoyama H et al. over-expression of Flt3 induces NF-κB pathway and increases the expression of IL6. Leuk Res 2005; 29: 893–899.
Schlessinger J . Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211–225.
Birkenkamp K, Geugien M, Schepers H, Westra J, Lemmink H, Vellenga E . Constitutive NF-kappaB DNA-bonding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia 2004; 18: 103–112.
Turco M, Romano M, Petrella A, Bisogni R, Tassone P, Venuta S . NF-kB/Rel mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia 2004; 18: 11–17.
Minami Y, Yamamoto K, Kiyoi H, Ueda R, Saito H, Naoe T . Different antiapoptotic pathways between wild-type and mutated FLT3: insights into therapeutic targets in leukemia. Blood 2003; 102: 2969–2975.
Cheng Q, Lee H, Li Y, Parks T, Cheng G . Upregulation of Bcl-x and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-κB inhibition. Oncogene 2000; 19: 4936–4940.
Acknowledgements
We thank Mrs Pascale Gaillard (Merck Serono) and Laura Liu (EMB-Serono) for their help with FLT3 experiments. This work was supported by institutional funding from INSERM, by grants from ARC, La Fondation de France comité leucémies and Merck Serono International SA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griessinger, E., Imbert, V., Lagadec, P. et al. AS602868, a dual inhibitor of IKK2 and FLT3 to target AML cells. Leukemia 21, 877–885 (2007). https://doi.org/10.1038/sj.leu.2404614
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404614
Keywords
This article is cited by
-
FLT3 inhibition: a moving and evolving target in acute myeloid leukaemia
Leukemia (2013)
-
Leukemia stem cells
Annals of Hematology (2011)
-
Sensitivity and gene expression profile of fresh human acute myeloid leukemia cells exposed ex vivo to AS602868
Cancer Chemotherapy and Pharmacology (2011)
-
Cancer stem cells and cancer therapy
Tumor Biology (2011)
-
Pristimerin induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation by blocking NF-κB signaling and depleting Bcr-Abl
Molecular Cancer (2010)