Abstract
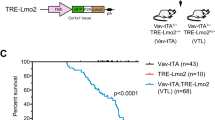
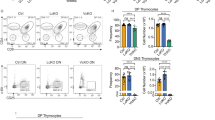
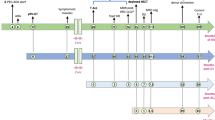
The occurrence of leukemia in a gene therapy trial for SCID-X1 has highlighted insertional mutagenesis as an adverse effect. Although retroviral integration near the T-cell acute lymphoblastic leukemia (T-ALL) oncogene LIM-only protein 2 (LMO2) appears to be a common event, it is unclear why LMO2 was preferentially targeted. We show that of classical T-ALL oncogenes, LMO2 is most highly transcribed in CD34+ progenitor cells. Upon stimulation with growth factors typically used in gene therapy protocols transcription of LMO2, LYL1, TAL1 and TAN1 is most prominent. Therefore, these oncogenes may be susceptible to viral integration. The interleukin-2 receptor gamma chain (IL2Rγ), which is mutated in SCID-X1, has been proposed as a cooperating oncogene to LMO2. However, we found that overexpressing IL2Rγ had no effect on T-cell development. In contrast, retroviral overexpression of LMO2 in CD34+ cells caused severe abnormalities in T-cell development, but B-cell and myeloid development remained unaffected. Our data help explain why LMO2 was preferentially targeted over many of the other known T-ALL oncogenes. Furthermore, during T-cell development retrovirus-mediated expression of IL2Rγ may not be directly oncogenic. Instead, restoration of normal IL7-receptor signaling may allow progression of T-cell development to stages where ectopic LMO2 expression causes aberrant thymocyte growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gamma retroviral vector. Lancet 2004; 364: 2181–2187.
Hacein-Bey-Abina S, Fischer A, Cavazzana-Calvo M . Gene therapy of X-linked severe combined immunodeficiency. Int J Hematol 2002; 76: 295–298.
Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193.
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Marshall E . Gene therapy. Second child in French trial is found to have leukemia. Science 2003; 299: 320.
Check E . Gene therapy put on hold as third child develops cancer. Nature 2005; 433: 561.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Dave UP, Jenkins NA, Copeland NG . Gene therapy insertional mutagenesis insights. Science 2004; 303: 333.
Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM . Gene therapy: therapeutic gene causing lymphoma. Nature 2006; 440: 1123.
Foot AB, Oakhill A, Kitchen C . Acute monoblastic leukemia of infancy in Klinefelter's syndrome. Cancer Genet Cytogenet 1992; 61: 99–100.
Lessard J, Sauvageau G . Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003; 423: 255–260.
Hussey DJ, Nicola M, Moore S, Peters GB, Dobrovic A . The (4;11)(q21;p15) translocation fuses the NUP98 and RAP1GDS1 genes and is recurrent in T-cell acute lymphocytic leukemia. Blood 1999; 94: 2072–2079.
Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM . Gene therapy: is IL2RG oncogenic in T-cell development?: X-SCID transgene leukaemogenicity (reply). Nature 2006; 443: E6–E7.
Shou Y, Ma Z, Lu T, Sorrentino BP . Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc Natl Acad Sci USA 2006; 103: 11730–11735.
Pike-Overzet K, de Ridder D, Weerkamp F, Baert MR, Verstegen MM, Brugman MH et al. Gene therapy: is IL2RG oncogenic in T-cell development? Nature 2006; 443: E6–E7, (E5 discussion).
Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EF, Baert MR et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med 2005; 201: 1715–1723.
van Hennik PB, Verstegen MM, Bierhuizen MF, Limon A, Wognum AW, Cancelas JA et al. Highly efficient transduction of the green fluorescent protein gene in human umbilical cord blood stem cells capable of cobblestone formation in long-term cultures and multilineage engraftment of immunodeficient mice. Blood 1998; 92: 4013–4022.
Wognum AW, Visser TP, Peters K, Bierhuizen MF, Wagemaker G . Stimulation of mouse bone marrow cells with kit ligand, FLT3 ligand, and thrombopoietin leads to efficient retrovirus-mediated gene transfer to stem cells, whereas interleukin 3 and interleukin 11 reduce transduction of short- and long-term repopulating cells. Hum Gene Ther 2000; 11: 2129–2141.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4: 249–264.
Bolstad BM, Irizarry RA, Astrand M, Speed TP . A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19: 185–193.
de Ridder D, Staal FJ, van Dongen JJ, Reinders MJ . Maximum significance clustering of oligonucleotide microarrays. Bioinformatics 2006; 22: 326–331.
Kinsella TM, Nolan GP . Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther 1996; 7: 1405–1413.
Weerkamp F, Baert MR, Brugman MH, Dik WA, de Haas EF, Visser TP et al. Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood 2006; 107: 3131–3137.
Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F . HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002; 110: 521–529.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2004; 2: E234.
Ng YY, van Kessel B, Lakhorst HM, Baert MR, van den Burg CM, Bloem AC et al. Gene expression profiling of CD34+ cells from various hematopoietic stem-cell sources reveals functional differences in stem-cell activity. J Leukoc Biol 2004; 75: 314–323.
Spits H, Blom B, Jaleco AC, Weijer K, Verschuren MC, van Dongen JJ et al. Early stages in the development of human T, natural killer and thymic dendritic cells. Immunol Rev 1998; 165: 75–86.
Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ et al. Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol 2005; 115: 834–840.
Puel A, Ziegler SF, Buckley RH, Leonard WJ . Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet 1998; 20: 394–397.
Roifman CM, Zhang J, Chitayat D, Sharfe N . A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood 2000; 96: 2803–2807.
Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J et al. Murine leukemia induced by retroviral gene marking. Science 2002; 296: 497.
Calmels B, Ferguson C, Laukkanen MO, Adler R, Faulhaber M, Kim HJ et al. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood 2005; 106: 2530–2533.
Weerkamp F, Pike-Overzet K, Staal FJ . T-sing progenitors to commit. Trends Immunol 2006; 27: 125–131.
Thrasher AJ, Gaspar HB, Baum C, Modlich U, Schambach A, Candotti F et al. Gene therapy: X-SCID transgene leukaemogenicity. Nature 2006; 443: E5–E6, (discussion E6–E7).
Larson RC, Osada H, Larson TA, Lavenir I, Rabbitts TH . The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene 1995; 11: 853–862.
Neale GA, Rehg JE, Goorha RM . Ectopic expression of rhombotin-2 causes selective expansion of CD4-CD8- lymphocytes in the thymus and T-cell tumors in transgenic mice. Blood 1995; 86: 3060–3071.
Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol 2003; 4: 168–174.
Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004; 306: 269–271.
Trono D . Virology. Picking the right spot. Science 2003; 300: 1670–1671.
Baum C, von Kalle C, Staal FJ, Li Z, Fehse B, Schmidt M et al. Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther 2004; 9: 5–13.
Acknowledgements
We thank Dr Langerak, Dr van Velden and Dr Dik for critically reading the manuscript. In addition, we are grateful for the primers and probes made available by Dr Dik. We also thank E de Haas for performing the sorting and Dr Kwee Yong, Department of Haematology, University College London, for supplying the PBSC. This work was supported in part by the 5th and 6th EU Framework program (Contract Nos. QLK3-CT-2001-0427 (INHERINET) and LSHB-CT-2004-005242 (CONSERT), as well as by the Translational Gene Therapy Research Programme of ZonMw – the Netherlands Organization for Health Research and Development. AJT is supported by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pike-Overzet, K., de Ridder, D., Weerkamp, F. et al. Ectopic retroviral expression of LMO2, but not IL2Rγ, blocks human T-cell development from CD34+ cells: implications for leukemogenesis in gene therapy. Leukemia 21, 754–763 (2007). https://doi.org/10.1038/sj.leu.2404563
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404563
Keywords
This article is cited by
-
A comprehensive analysis of LMO2 pathogenic regulatory profile during T-lineage development and leukemic transformation
Oncogene (2022)
-
LMO2 and IL2RG synergize in thymocytes to mimic the evolution of SCID-X1 gene therapy-associated T-cell leukaemia
Leukemia (2016)
-
Stem Cell Leukemia: how a TALented actor can go awry on the hematopoietic stage
Leukemia (2016)
-
The non-canonical Wnt receptor Ryk regulates hematopoietic stem cell repopulation in part by controlling proliferation and apoptosis
Cell Death & Disease (2016)
-
Novel TAL1 targets beyond protein-coding genes: identification of TAL1-regulated microRNAs in T-cell acute lymphoblastic leukemia
Leukemia (2013)