Abstract
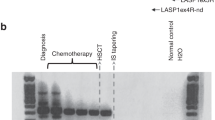
Evaluation of molecular response is important for the diagnosis and monitoring of minimal residual disease in patients with acute promyelocytic leukemia (APL). In this study, we analyzed the molecular response by regular reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time RT-PCR in 31 newly diagnosed patients. The real-time RT-PCR results are reported as normalized DoseN and log-reduction (3.0–4.9 log-reduction as minor and ⩾5.0 log-reduction as major molecular response). After induction therapy and completion of consolidation, minor molecular response was documented in 35.5 and 96.8% patients, respectively, which was equivalent to the regular RT-PCR (22.6 and 96.8%), whereas the major molecular response rate was significantly lower (12.9 and 90.3%, respectively). All patients achieved major molecular response during and after maintenance therapy. During the follow-up study, loss of major molecular response was observed in two patients, which was associated with subsequent loss of minor molecular response, positive RT-PCR and then documentation of central nervous system leukemia or clinical relapse in 3–6 months. For summary, we demonstrated that the real-time RT-PCR is potentially superior to regular RT-PCR in evaluation of molecular response in APL patients and that reporting real-time RT-PCR data by log-reduction is feasible and clinically relevant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Avvisati G, Lo Coco F, Mandelli F . Acute promyelocytic leukemia: clinical and morphologic features and prognostic factors. Semin Hematol 2001; 38: 4–12.
Huang ME, Ye YC, Chen SR, Chai JR, Zhoa L, Wang ZY . Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988; 72: 567–572.
Fenaux P, Chomienne C, Degos L . All-trans retinoic acid and chemotherapy in the treatment of acute promyelocytic leukemia. Semin Hematol 2001; 38: 13–25.
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I.As2O3 exerts dose-dependent dual effects on APL cells. Blood 1997; 89: 3345–3353.
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 1998; 339: 1341–1348.
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 1996; 88: 1052–1061.
Lallemand-Breitenbach V, Guillemin MC, Janin A, Daniel MT, Degos L, Kogan SC et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med 1999; 189: 1043–1052.
Jing Y, Wang L, Xia L, Chen GQ, Chen Z, Miller WH et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood 2001; 97: 264–269.
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA 2004; 101: 5328–5335.
Castaigne S, Balitrand N, de The H, Dejean A, Degos L, Chomienne C . A PML/retinoic acid receptor alpha fusion transcript is constantly detected by RNA-based polymerase chain reaction in acute promyelocytic leukemia. Blood 1992; 79: 3110–3115.
Hu J, Shen ZX, Sun GL, Chen SJ, Wang ZY, Chen Z . Long-term survival and prognostic study in acute promyelocytic leukemia treated with all-trans-retinoic acid, chemotherapy, and As2O3: an experience of 120 patients at a single institution. Int J Hematol 1999; 70: 248–260.
Gameiro P, Vieira S, Carrara P, Silva AL, Diamond J, Botelho de Sousa A et al. The PML-RAR alpha transcript in long-term follow-up of acute promyelocytic leukemia patients. Haematologica 2001; 86: 577–585.
Martinelli G, Remiddi C, Visani G, Farabegoli P, Testoni N, Zaccaria A et al. Molecular analysis of PML-RAR alpha fusion mRNA detected by reverse transcription-polymerase chain reaction assay in long-term disease-free acute promyelocytic leukaemia patients. Br J Haematol 1995; 90: 966–968.
Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter ‘AIDA’ trial. GIMEMA-AIEOP Multicenter ‘AIDA’ Trial. Blood 1998; 92: 784–789.
Mandelli F, Diverio D, Avvisati G, Luciano A, Barbui T, Bernasconi C et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood 1997; 90: 1014–1021.
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood 1999; 94: 2225–2229.
Grimwade D, Lo Coco F . Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia 2002; 16: 1959–1973.
Cassinat B, Zassadowski F, Balitrand N, Barbey C, Rain JD, Fenaux P et al. Quantitation of minimal residual disease in acute promyelocytic leukemia patients with t(15;17) translocation using real-time RT-PCR. Leukemia 2000; 14: 324–328.
Gu BW, Hu J, Xu L, Yan H, Jin WR, Zhu YM et al. Feasibility and clinical significance of real-time quantitative RT-PCR assay of PML-RARalpha fusion transcript in patients with acute promyelocytic leukemia. Hematol J 2001; 2: 330–340.
Gallagher RE, Yeap BY, Livak KJ, Beaubier N, Bloomfield CD, Appelbaum FR et al. Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood 2003; 101: 2521–2528.
Huang W, Sun GL, Li XS, Cao Q, Lu Y, Jang GS et al. Acute promyelocytic leukemia: clinical relevance of two major PML-RAR alpha isoforms and detection of minimal residual disease by retrotranscriptase/polymerase chain reaction to predict relapse. Blood 1993; 82: 1264–1269.
Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Koheseisho). Blood 1999; 93: 3074–3080.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV et al. Design and analysis of randomized clinical trials requiring prolonged observations of each patient, II: Analysis and examples. Br J Cancer 1977; 35: 1–39.
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH . Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood 1999; 93: 4131–4143.
Jurcic JG, Nimer SD, Scheinberg DA, DeBlasio T, Warrell Jr RP, Miller Jr WH . Prognostic significance of minimal residual disease detection and PML/RAR-α isoform type: long-term follow-up in acute promyelocytic leukemia. Blood 2001; 98: 2651–2656.
Grimwade D, Howe K, Langabeer S, Burnett A, Goldstone A, Solomon E . Minimal residual disease detection in acute promyelocytic leukemia by reverse-transcriptase PCR: evaluation of PML-RARα and RARα-PML assessment in patients who ultimately relapse. Leukemia 1996; 10: 61–66.
Lo Coco F, Diverio D, Pandolfi PP, Biondi A, Rossi V, Avvisati G et al. Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukaemia. Lancet 1992; 340: 1437–1438.
Jurcic JG . Monitoring PML-RARalpha in acute promyelocytic leukemia. Curr Oncol Rep 2003; 5: 391–398.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – a Europe Against Cancer program. Leukemia 2003; 7: 2318–2357.
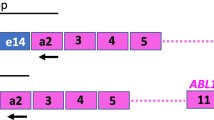
Chen SJ, Chen Z, Chen A, Tong JH, Dong S, Wang ZY et al. Occurrence of distinct PML-RAR-alpha fusion gene isoforms in patients with acute promyelocytic leukemia detected by reverse transcriptase/polymerase chain reaction. Oncogene 1992; 7: 1223–1232.
Paietta E, Goloubeva O, Neuberg D, Bennett JM, Gallagher R, Racevskis J, et al., Eastern Cooperative Oncology Group. A surrogate marker profile for PML/RAR alpha expressing acute promyelocytic leukemia and the association of immunophenotypic markers with morphologic and molecular subtypes. Cytometry B Clin Cytom 2004; 59: 1–9.
Vahdat L, Maslak P, Miller Jr WH, Eardley A, Heller G, Scheinberg DA et al. Early mortality and retinoic acid syndrome in acute promyelocytic leukemia: impact of leukocytosis, low-dose chemotherapy, PML/RARα isoform, and CD13 expression in patients treated with all-trans retinoid acid. Blood 1994; 84: 3843–3849.
Gallagher R, Willman CL, Slack JL, Andersen JW, Li YP, Viswanatha D et al. Association of PML/RARα fusion mRNA type with pretreatment hematologic characteristics but not treatment outcome in acute promyelocytic leukemia: an intergroup molecular study. Blood 1997; 90: 1656–1663.
Kiyoi H, Naoe T . FLT3 in human hematologic malignancies. Leuk Lymphoma 2002; 43: 1541–1547.
Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood 2004; 103: 1901–1908.
Noguera NI, Breccia M, Divona M, Diverio D, Costa V, De Santis S et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia 2002; 16: 2185–2189.
Chillon MC, Fernandez C, Garcia-Sanz R, Balanzategui A, Ramos F, Fernandez-Calvo J et al. FLT3-activating mutations are associated with poor prognostic features in AML at diagnosis but they are not an independent prognostic factor. Hematol J 2004; 5: 239–246.
Au WY, Fung A, Chim CS, Lie AK, Liang R, Ma ES et al. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol 2004; 125: 463–469.
Acknowledgements
Grants and financial support: This work was supported in part by Grants from Co-Pi Program of Samuel-Waxman Cancer Research Foundation, Chinese National High Tech Program (863), National Key Program for Basic Research (973), National Natural Science Foundation of China, Shanghai Municipal Commission for Education (Y0201) and the Shanghai Municipal Commission for Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YF., Zhu, YM., Shen, SH. et al. Molecular response in acute promyelocytic leukemia: a direct comparison of regular and real-time RT-PCR. Leukemia 20, 1393–1399 (2006). https://doi.org/10.1038/sj.leu.2404262
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404262
Keywords
This article is cited by
-
Diagnosis and treatment of acute promyelocytic leukemia
Current Oncology Reports (2007)