Abstract
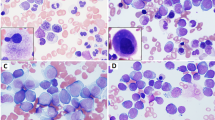
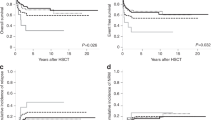
We report a retrospective analysis of children with myelodysplastic syndrome (MDS) diagnosed between 1990 and 1997 in Japan. In total, 189 patients were enrolled: 122 cases of primary MDS (26 RA, 18 RAEB, 25 RAEBt, 53 CMML/JMML), 24 cases with constitutional predisposition to MDS, and 43 cases of therapy-related MDS (t-MDS). The frequency of pediatric MDS was estimated to be 7.7% of all leukemias. Cytogenetic abnormalities were observed in 41% of primary MDS and 90% of t-MDS cases. The 4-year survival rate, estimated by Kaplan–Meier analysis, for primary RA was 78.9%, while other types of MDS and JMML had rates lower than 40%, and t-MDS showed an even more unfavorable prognosis. In primary MDS, the survival rate of patients with cytogenetic abnormalities was significantly lower. Among prognostic variables by IPSS, only the cytogenetic pattern was useful for predicting outcome in childhood MDS. There was no apparent advantage to chemotherapy for RA, and the survival rate in patients with primary RA, JMML, or t-MDS receiving stem cell transplantation was significantly higher. More precise designs of our diagnostic and classification systems, as well as therapeutic trials in large-scale prospective studies, are necessary for further improvements in MDS outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hasle H, Jacobsen BB, Pedersen NT . Myelodysplastic syndrome in childhood: a population based study of nine cases Br J Haematol 1992 81: 495–498
Tuncer MA, Pagliuca A, Hicsonmez G, Yetgin S, Ozsoylu S, Mufti GJ . Primary myelodysplastic syndrome in children: the clinical experience in 33 cases Br J Haematol 1992 82: 347–353
Creutzig U, Cantu-Rajnoldi A, Ritter J, Romitti L, Odenwald E, Conter V, Riehm H, Masera G . Myelodysplastic syndrome in childhood. Report of 21 patients from Italy and West Germany Am J Pediat Hematol/Oncol 1987 9: 324–330
Hasle H, Kerndrup G, Jacobsen BB . Childhood myelodysplastic syndrome in Denmark: incidence and predisposing conditions Leukemia 1995 9: 1569–1572
Bader-Meunier B, Mielot F, Tchernia G, Busine J, Delsol G, Duchayne E, Lemerle S, Leverger G, Lumley LD, Manel AM, Nathanson M, Plantaz D, Robert A, Schaison G, Sommelet D, Vilmer E . Myelodysplastic syndrome in childhood: report of 49 patients from a French multicentre study Br J Haematol 1996 92: 344–350
Luna-Fineman S, Shannon KM, Atwater SK, Davis J, Masterson M, Ortega G, Sanders J, Steinherz P, Weinberg V, Lange BJ . Myelodysplastic and myeloproliferative disorders of childhood: a study of 167 patients Blood 1999 93: 459–466
Passmore SJ, Hann IM, Stiller CA, Ramani P, Swansbury GJ, Gibbons B, Reeves BR, Chessells JM . Pediatric myelodysplasia: a study of 68 children and a new prognostic scoring system Blood 1995 85: 1742–1750
Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR . A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada Br J Haematol 1999 106: 1027–1032
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, Gralnick HR, Sultan C . Proposal for the classification of the myelodysplastic syndromes Br J Haematol 1982 51: 189–199
Arico M, Biondi A, Pui CH . Juvenile myelomonocytic leukemia Blood 1997 90: 479–488
Niemeyer CM, Fenu S, Hasle H, Mann G, Stary J, Wering E . Differentiating juvenile myelomonocytic leukemia from infectious disease Blood 1998 91: 365–367
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J . International scoring system for evaluating prognosis in myelodysplastic syndrome Blood 1997 89: 2079–2088
Lange BJ, Kobrinsky N, Barnard DR, Arthur DC, Buckley JD, Howells WB, Gold S, Sanders J, Neudorf S, Smith FO, Woods WG . Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children's Cancer Group Studies 2861 and 2891 Blood 1998 91: 608–615
Shannon KM, Watterson J, Johnson P, O'Connell P, Lange B, Shah N, Steinherz P, Kan KW, Priest JR . Monosomy 7 myeloproliferative disease in children with neurofibromatosis, type 1: epidemiology and molecular analysis Blood 1992 79: 1311–1318
Auerbach A, Allen R . Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry Cancer Genet Cytogenet 1991 51: 1–12
Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K . Loss of NF-1 results in activation of the RAS signaling pathway and leads to aberrant growth in haematopoietic cells Nat Genet 1996 12: 144–148
Side L, Taylor B, Cayouette M, Conner E, Thompson P, Luce M, Shannon K . Homozygous inactivation of the NF-1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders N Engl J Med 1997 336: 1713–1720
Guba SC, Sartor CA, Hutchinson R, Boxer LA, Emerson SG . Granulocyte colony-stimulating factor (G-CSF) production and G-CSF receptor structure in patients with congenital neutropenia Blood 1992 83: 1486–1492
Kalra R, Dale D, Freedman M, Bonilla MA, Weinblatt M, Ganser A, Bowman P, Abish S, Priest J, Oseas RS, Olson K, Paderanga D, Shannon K . Monosomy 7 and activating RAS mutations accompany malignant transformation in patients with congenital neutropenia Blood 1995 86: 4579–4586
De Planque MM, Bacigalupo A, Wursch A, Hows JM, Devergie A, Frickhofen N, Brand A, Nissen K . Long-term follow-up of severe aplastic anaemia patients treated with antithymocyte globlin Br J Haematol 1989 73: 121–126
Tichelli A, Gratwohl A, Wursch A, Nissen C, Speck B . Late haematological complications in severe aplastic anemia Br J Haematol 1988 69: 413–418
Tichelli A, Gratwohl A, Nissen C, Signer E, Gysi CS, Speck B . Morphology in patients with severe aplastic anemia treated with antilymphocyte globlin Blood 1992 80: 337–345
Ohara A, Kojima S, Hamajima N, Tsuchida M, Imashuku S, Ohta S, Sasaki H, Okamura J, Sugita K, Kigasawa H, Kiriyama Y, Akatsuka J, Tsukimoto I . Myelodysplastic syndrome and acute myelogenous leukemia as a late clonal complication in children with acquired aplastic anemia Blood 1997 90: 1009–1013
Marsh JCW, Geary CG . Is aplastic anemia a pre-leukemic disorder? Br J Haematol 1991 77: 447–452
Luna-Fineman S, Shannon KM, Lange BJ . Childhood monosomy 7: epidemiology, biology, and mechanistic implications Blood 1995 85: 1985–1999
Appelbaum FR, Barrall J, Storb R, Ramberg R, Doney K, Sale GE, Thomas D . Clonal cytogenetic abnormalities in patients with otherwise typical aplastic anemia Exp Hematol 1987 15: 1134–1139
Le Beau MML, Espinosa R, Davis EM, Eisenbart JD, Larson RA, Green ED . Cytogenetic and molecular delineation of a region of chromosome 7 commonly deleted in malignant myeloid diseases Blood 1996 88: 1930–1935
Fischer K, Frohling S, Scherer SW, Brown JM, Scholl C, Stilgenbauer S, Tsui LC, Lichter P, Dohner H . Molecular cytogenetic delineation of deletions and translocations involving chromosome band 7q22 in myeloid leukemias Blood 1997 89: 2036–2041
Neubauer A, Shannon K, Liu E . Mutation of the ras proto-oncogene in childhood monosomy 7 Blood 1991 77: 594–598
Hasle H, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Fenu S, Fonattsch C, Haas OA, Harbott J, Kardos G, Kerndrup G, Mann G, Niemeyer CM, Ptoszkova H, Ritter J, Slater R, Stary J, Stollmann-Gibbels B, Testi AM, van Wernig ER, Zimmermann M . Myelodysplastic syndrome, juvenile myelomonocytic leukemia, and acute myeloid leukemia associated with complete or partial monosomy 7 Leukemia 1999 13: 376–385
Locatelli F, Zecca M, Niemeyer C, Angelucci E, Arcese G, Bender-Gotze C, Bonetti F, Burdach S, Dini G, Ebell W, Friedrich W, Hasle H, Herman J, Jacobsen N, Klingebiel T, Kremens B, Mann G, Miniero R, Pession A, Peters C, Paolucci P, Rossetti F, Schmid HJ, Stary J, Suttorp M, Uderzo C, Veer-Korthof ETV, Vossen J, Zimmermann M . Role of allogeneic bone marrow transplantation for the treatment of myelodysplastic syndromes in childhood Bone Marrow Transplant 1996 18 (Suppl. 2): 63–67
Locatelli F, Niemeyer C, Angelucci E, Bender-Gotze C, Burdach S, Ebell W, Friedrich W, Hasle H, Herman J, Jacobsen N, Klingebiel T, Kremens B, Mann G, Pession A, Peters C, Schmid HJ, Stary J, Suttorp M, Uderzo C, Veer-Korthof ETV, Vossen J, Zecca M, Zimmermann M . Allogeneic bone marrow transplantation for chronic myelomonocytic leukemia in childhood: a report from the European Working Group on Myelodysplastic Syndrome in Childhood J Clin Oncol 1997 15: 566–573
Acknowledgements
The authors express sincere gratitude to the members of Japan Pediatric Hematology Society for their cooperation in this study. We also thank Dr Ichiro Tsukimoto for providing the data of registry on childhood leukemia and aplastic anemia.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Sasaki, H., Manabe, A., Kojima, S. et al. Myelodysplastic syndrome in childhood: a retrospective study of 189 patients in Japan. Leukemia 15, 1713–1720 (2001). https://doi.org/10.1038/sj.leu.2402271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402271
Keywords
This article is cited by
-
Radiation-sparing reduced-intensity unrelated umbilical cord blood transplantation for rare hematological disorders in children
International Journal of Hematology (2022)
-
Improved outcomes of allogeneic hematopoietic stem cell transplantation including haploidentical transplantation for childhood myelodysplastic syndrome
Bone Marrow Transplantation (2020)
-
The genomic landscape of pediatric myelodysplastic syndromes
Nature Communications (2017)
-
Pediatric myelodysplastic syndromes
Journal of Hematopathology (2015)
-
Cytogenetics and clinical features of pediatric myelodysplastic syndrome in Japan
International Journal of Hematology (2014)