Abstract
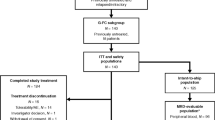
Between May 1987 and January 1991, 1354 patients, 1–21 years old, with standard or poor prognosis B-lineage acute lymphocytic leukemia were treated on the Pediatric Oncology Group Study 8602. One thousand three hundred and twenty-three patients entered remission and 1051 patients were randomized on day 43 to an intensification regimen containing L-asparaginase and intermediate-dose methotrexate (regimen B) or cytarabine and intermediate dose methotrexate (regimen C). After completion of intensification at week 25, all patients received the same maintenance therapy until 3 years from diagnosis. Overall 5-year continuous complete remission (CCR) for regimen B was 72 ± 2% (s.e.) and for regimen C, 73 ± 2% (P = 0.72 by log-rank analysis). Significant differences between treatments for CCR, testicular, CNS relapses overall or with regard to phenotype (pre-B vs early pre-B), gender, or race were not detected. During intensification, regimen C had significantly more bacterial infections (P = 0.05) and days spent in the hospital (P < 0.001) compared with regimen b, while regimen b had significantly more allergic reactions (P < 0.0001). no significant differences in ccr were noted between patients with pre-b and early pre-b all (P = 0.22 stratified by risk group and treatment). This study was unable to detect statistical difference between asparaginase (regimen B) and cytarabine (regimen C) during the intensification phase of therapy in children with B-lineage acute lymphocytic leukemia. Leukemia (2000) 14, 1570–1576.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, Odenwald D, Ritter J, Mann G, Welte K, Gadner H, Riehm H . Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86 Blood 1994 84: 3122–3133
Chessells JM, Bailey C, Richards SM . Intensification of treatment and survival in all children with lymphoblastic leukaemia: results of UK Medical Research Council trial UKALL X. Medical Research Council Working Party on Childhood Leukaemia Lancet 1995 345: 143–148
Sinks LF, Wang JJ, Freeman AI . The treatment of primary childhood acute lymphocytic leukemia with intermediate dose methotrexate Hamatol Bluttransfus 1981 26: 99–107
Green DM, Brecher ML, Blumenson LE, Grossi M, Freeman AI . The use of intermediate dose methotrexate in increased risk childhood acute lymphoblastic leukemia Cancer 1982 50: 2722–2727
Freeman AI, Weinberg V, Brecher ML, Jones B, Glicksman AS, Sinks LF, Weil M, Pleuss H, Hananian J, Burgert EO Jr, Gilchrist GS, Necheles T, Harris M, Kung F, Patterson RB, Maurer H, Leventhal B, Chevalier L, Forman E, Holland JF . Comparison of intermediate-dose methotrexate with cranial irradiation for the post-induction treatment of acute lymphocytic leukemia in children New Engl J Med 1983 308: 477–484
Freeman AI, Boyett JM, Glicksman AS, Brecher ML, Leventhal BG, Sinks LF, Holland JF . Intermediate-dose methotrexate versus cranial irradiation in childhood acute lymphoblastic leukemia: a ten-year follow-up Med Pediatr Oncol 1997 28: 98–107
Krance RA, Newman EM, Ravindranath Y, Harris MB, Brecher M, Wimmer R, Shuster JJ, Land VJ, Pullen J, Crist W, Pinkel D . A pilot study of intermediate-dose methotrexate and cytosine arabinoside, ‘spread-out’ or ‘up-front’, in continuation therapy for childhood non-T, non-B acute lymphoblastic leukemia. A Pediatric Oncology Group study Cancer 1991 67: 550–556
Clavell LA, Gelber RD, Cohen HJ, Hitchcock-Bryan S, Cassady JR, Tarbell NJ, Blattner SR, Tantravahi R, Leavitt P, Sallan SE . Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia New Engl J Med 1986 315: 657–663
Graubner UB, Haas RJ, Janka G, Gaedicke G, Kohne E, Rieber EP . The Munich study on the treatment of acute lymphoblastic leukemia in childhood (ALL 77–02) Klin Padiatr 1985 197: 207–214
Land VJ, Shuster JJ, Crist WM, Ravindranath Y, Harris MB, Krance RA, Pinkel D, Pullen DJ . Comparison of two schedules of intermediate-dose methotrexate and cytarabine intensification therapy for childhood B-precursor cell acute lymphoblastic leukemia: a Pediatric Oncology Group study J Clin Oncol 1994 12: 1939–1945
Harris MB, Shuster JJ, Pullen DJ, Borowitz MJ, Carroll AJ, Behm FG, Land VJ . Intensification therapy with antimetabolite-based therapy in standard-risk acute lymphocytic leukemia of childhood: a Pediatric Oncology Group study J Clin Oncol 1998 16: 2840–2847
Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reaman G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R . Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia J Clin Oncol 1996 14: 18–24
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG . Design and analysis of randomized clinical trials requiring prolonged observation of each patient Br J Cancer 1977 35: 1–39
Kaplan EL, Meier P . Nonparametric estimation form incomplete observations J Am Stat Assoc 1958 53: 457–481
Shuster JJ . Fixing number of events in large comparative trials with low event rates: a binomial approach Control Clin Trials 1993 14: 198–208
Schorin MA, Blattner S, Gelber RD, Tarbell NJ, Donnelly M, Dalton V, Cohen HJ, Sallan SE . Treatment of acute lymphoblastic leukemia: results of Dana-Farber Cancer Institute/Children’s Hospital Acute Lymphoblastic Leukemia Consortium Protocol 85–01 J Clin Oncol 1994 12: 740–747
Pui C-H . Childhood leukemias New Engl J Med 1995 332: 1618–1630
Kamps WA, Bokkerink JP, Hahlen K, Hermans J, Riehm H, Gadner H, Schrappe M, Slater R, van den Berg-de Ruiter E, Smets LA, de Vaan GA, Weening RS, van Weerden JF, van Wering ER, den der Does-van den Berg A . Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: results of Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988–1991) Blood 1999 94: 1226–1236
Kurtzberg J, Asselin B, Poplack D, Grebanier A, Chen R, Franklin A, Scudiery D, Fisherman J . Antibodies to asparaginase alter pharmacokinetics and decrease enzyme activity in patients on asparaginase therapy (Meeting abstract) Proc Annu Meet Am Assoc Cancer Res 1993 34: A1807
Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ . Comparative pharmacokinetic studies of three asparaginase preparations J Clin Oncol 1993 11: 1780–1786
Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, Rubnitz JE, Harrison PL, Wang B, Evans WE, Pui C-H, Relling MV . Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia J Clin Oncol 2000 18: 1525–1532
Jackson RC, Hardkrader RJ . Synergistic and antagonistic interactions of methotrexate and 1-β-D-arabinofuranosylcytosine in hepatoma cells: the modulating effect of purines Biochem Pharmacol 1981 30: 223–229
Newman EM, Villacorte DG, Testi AM, Krance RA, Harris MB, Ravindranath Y, Pinkel D . Biochemical interactions between methotrexate and 1-beta-D-arabinofuranosylcytosine in hematopoietic cells of children: a Pediatric Oncology Group study Cancer Chemother Pharmacol 1990 27: 60–66
Cadman E, Eifman F . Mechanism of synergistic cell killing when methotrexate precedes cytarabine: study of L1210 and human leukemic cells J Clin Invest 1979 64: 788–797
Graham ML, Shuster JJ, Kamen BA, Land VJ, Borowitz MJ, Camitta B, Cheo DL, Harrison MP, Leventhal BG, Pinkel DP, Pullen DJ, Steuber P, Whitehead VM . Changes in red blood cell methotrexate pharmacology and their impact on outcome when cytarabine is infused with methotrexate in the treatment of acute lymphocytic leukemia in children: a Pediatric Oncology Group study Clin Cancer Res 1996 2: 331–337
WM Crist, AJ Carroll, JJ Shuster, FG Behm, M Whitehead, TJ Vietti, AT Look, D Mahoney, A Ragab, DJ Pullen . Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): a Pediatric Oncology Group study Blood 1990 76: 117–122
SC Raimondi, FG Behm, PK Roberson, DL Williams, CH Pui, WM Crist, AT Look, Rivera GK . Cytogenetics of pre-B-cell acute lymphoblastic leukemia with emphasis on prognostic implications of the t(1;19) J Clin Oncol 1990 8: 1380–1388
Uckun FM, Sensel MG, Sather HN, Gaynon PS, Arthur DC, Lange BJ, Steinherz PG, Kraft P, Hutchinson R, Nachman JB, Reaman GJ, Heerema NA . Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report of the Children’s Cancer Group J Clin Oncol 1998 16: 527–535
Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS . Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy New Engl J Med 1998 338: 1663–1671
Acknowledgements
This work was supported in part by The Tomorrows Children’s Fund and National Cancer Institute Grant CA-35096.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harris, M., Shuster, J., Pullen, J. et al. Treatment of children with early pre-B and pre-B acute lymphocytic leukemia with antimetabolite-based intensification regimens: a Pediatric Oncology Group Study. Leukemia 14, 1570–1576 (2000). https://doi.org/10.1038/sj.leu.2401886
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401886
Keywords
This article is cited by
-
Randomized assessment of delayed intensification and two methods for parenteral methotrexate delivery in childhood B-ALL: Children’s Oncology Group Studies P9904 and P9905
Leukemia (2020)
-
Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the children's oncology group
Leukemia (2010)